Synthesis of tertiary alkyl amines via photoinduced copper-catalysed nucleophilic substitution
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
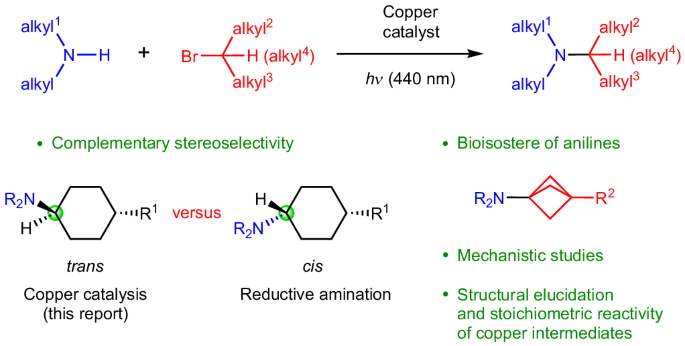
In view of the high propensity of tertiary alkyl amines to be bioactive, the development of new methods for their synthesis is an important challenge. Transition-metal catalysis has the potential to greatly expand the scope of nucleophilic substitution reactions of alkyl electrophiles; unfortunately, in the case of alkyl amines as nucleophiles, only one success has been described so far: the selective mono-alkylation of primary amines to form secondary amines. Here, using photoinduced copper catalysis, we report the synthesis of tertiary alkyl amines from secondary amines and unactivated alkyl electrophiles, two readily available coupling partners. Utilizing an array of tools, we have analysed the mechanism of this process; specifically, we have structurally characterized the three principal copper-based intermediates that are detected during catalysis and provided support for the key steps of the proposed catalytic cycle, including the coupling of a copper(II)–amine intermediate with an alkyl radical to form a C–N bond. Alkyl amines are found in a wide array of bioactive compounds, making strategies for their synthesis very important. Now, a method has been developed to synthesize tertiary alkyl amines via photoinduced, copper-catalysed nucleophilic substitution of unactivated alkyl halides by secondary alkyl amines, with key copper intermediates elucidated in a detailed mechanistic study.

光诱导铜催化亲核取代合成叔烷基胺
鉴于叔烷基胺具有较高的生物活性,开发新的合成方法是一项重要的挑战。过渡金属催化具有极大扩展烷基亲电试剂亲核取代反应范围的潜力;不幸的是,在烷基胺作为亲核试剂的情况下,迄今为止只有一个成功的描述:伯胺的选择性单烷基化形成仲胺。在这里,我们报道了利用光诱导铜催化,从仲胺和未活化的烷基亲电试剂这两个容易获得的偶联伙伴合成叔烷基胺。利用一系列工具,我们分析了这一过程的机制;具体来说,我们对催化过程中检测到的三个主要铜基中间体进行了结构表征,并为所提出的催化循环的关键步骤提供了支持,包括铜(II) -胺中间体与烷基自由基的偶联形成C-N键。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


