Versatile Stimuli-Responsive Controlled Release of Pinanediol-Caged Boronic Esters for Spatiotemporal and Nitroreductase-Selective Glucose Bioimaging
IF 8.2
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
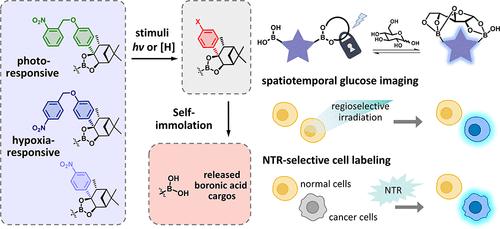
Boronic acids have been widely applied in various biological fields, particularly achieving significant practical progress in boronic acid–based glucose sensing. However, boronic acids exhibit nonspecific binding to other nucleophiles, and the inherent lability of boronic esters in biological systems limits their further applications. Herein, we developed a stimuli-responsive controllable caging strategy to achieve photoresponsive spatiotemporally and nitroreductase-responsive cancer cell-selective glucose sensing. We introduced o-/p-nitroaryl-containing self-immolative linkers onto δ-pinanediol derivatives, effectively caging boronic acids and blocking glucose recognition. Upon triggering by specific stimuli, the caged boronic esters decompose, releasing boronic acids and thereby restoring glucose recognition of the diboronic acid–based sensor. The proof of concept was confirmed through intracellular glucose bioimaging in living cells. Upon regional UV irradiation, we could monitor intracellular glucose with excellent spatiotemporal selectivity. Furthermore, we used the cancer biomarker nitroreductases as the internal stimuli and utilized the caged glucose sensor to selectively label hypoxic cancer cells in a cocultured living cell sample. We believe that our stimuli-responsive caging strategies will hold promising potential for the controlled release of other boronic acids in various biological contexts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sensors
Chemical Engineering-Bioengineering
CiteScore
14.50
自引率
3.40%
发文量
372
期刊介绍:
ACS Sensors is a peer-reviewed research journal that focuses on the dissemination of new and original knowledge in the field of sensor science, particularly those that selectively sense chemical or biological species or processes. The journal covers a broad range of topics, including but not limited to biosensors, chemical sensors, gas sensors, intracellular sensors, single molecule sensors, cell chips, and microfluidic devices. It aims to publish articles that address conceptual advances in sensing technology applicable to various types of analytes or application papers that report on the use of existing sensing concepts in new ways or for new analytes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


