Synergistic Hydrothermal Conversion of Biomass Derivative Carbohydrates and CO2 into Value-Added Organic Acid over an NH2-MIL-53(Fe) Catalyst
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
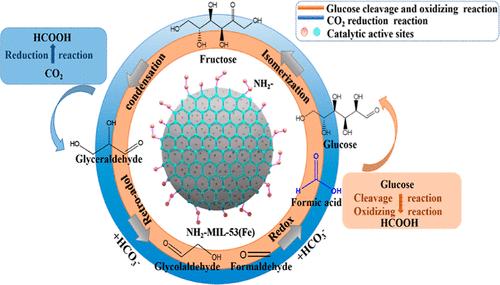
During CO2/HCO3– reduction with renewable biomass, achieving high-efficiency production of the target products is vital but challenging. In this study, an NH2– functional-group-modified MIL-53(Fe) catalyst was synthesized using a facile and efficient method. Under the action of the NH2-MIL-53(Fe) catalyst, a clear synergistic effect was exhibited on the transformation of glucose and NaHCO3 into formic acid with a high yield of 50% at a low reaction temperature (190 °C) via a three-pronged route, which mainly involved the decomposition of intermediates of glucose to gradually reduce NaHCO3 to formic acid under hydrothermal conditions. An in-depth analysis of the catalytic mechanism and density functional theory calculations demonstrated that the increased alkalinity of active sites by the NH2– functional group incorporation into the catalytic system enhanced the crucial reaction steps and reduced the activation energy of the crucial reactions, including glucose isomerization, aldehyde intermediate retro-aldol condensation, and redox of aldehyde compounds and NaHCO3 to formic acid, thereby promoting the generation of target products and suppressing side products. This study addresses the challenge of reducing NaHCO3 from renewable biomass to commercial formic acid by constructing multifunctional active sites, thus providing a new strategy for achieving carbon cycling.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
文献相关原料
公司名称
产品信息
阿拉丁
1,3-dihydroxyacetone dimer
阿拉丁
pyruvaldehyde
阿拉丁
acetic acid
阿拉丁
formic acid
阿拉丁
d(+)-Glucose
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


