Tuning Surface Valences of Nanoengagers to Enhance Their Structural Advantages for Efficiently Redirecting T Cells against Solid Tumors
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
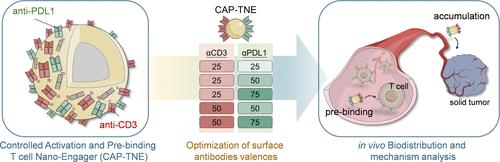
The nanoengager strategy, which enhances receptor signaling responsiveness through a multivalent ligand binding mode, offers a promising approach for improving immune cell redirecting therapy. Increasing nanomaterial platforms have been developed for constructing more flexible and multifunctional nanoengagers, but the different mediating mechanisms from their multivalent nanostructures, compared to original monomolecule engagers, have rarely been discussed. Here, we constructed dual-specificity T cell nanoengagers (TNEs) targeting CD3 and PDL1 receptors based on a polyethylene glycol-b-polylactic acid (PEG-b-PLA)-assembled nanoparticle and specifically studied the impact of surface antibody valences on their functional mechanisms, thereby enhancing the structural advantages of TNEs against solid tumors. Major conclusions include the following: (1) Valence control of surface antibodies is crucial for ensuring the safety and efficacy of TNEs when redirecting T cells. (2) Tuning the valence ratios of the two antibodies with the respective targeting is essential for enhancing the tumor-targeting capability and overall antitumor efficacy of TNEs, while TNE with a valence ratio of anti-CD3 to anti-PDL1 of 25:50 demonstrated the best performance. (3) Compared to monovalent soluble engager molecules, TNEs with an optimized surface multivalent antibody structure can effectively promote the enrichment of peripheral T cells into solid tumor sites and improve the tumor immune microenvironment. These results demonstrate the importance of optimizing the surface antibody valences to enhance the structural advantages of the TNE and would benefit the design and application of nanoparticle-based engagers, especially for TNEs.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


