Heteropolyacid Ligands in Two-Dimensional Channels Enable Lithium Separation from Monovalent Cations
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Extracting lithium from salt lakes requires ion-selective membranes with customizable nanochannels. However, it remains a major challenge to separate alkali cations due to their same valences and similar ionic radius. Inspired by the K+ channel of KcsA K+, significant progress has been made in adjusting nanochannel size to control the ion selectivity dominated by alkali cations dehydration. Besides, several works involved incorporating ligands, such as crown ether, into nanochannels based on coordination chemistry to try to promote alkali cation selectivity; nevertheless, only the separation between mono-/bivalent cations has been achieved. Herein, a series of heteropolyacid (HPA) ligands are designed to functionalize two-dimensional (2D) nanochannels, achieving superior lithium perm-selectivity over other alkali cations (16 for Li+/K+), with the Li+ permeation rate increased to four times that of the pristine 2D membrane. We discover that the switching of an ion between its hydration and ion-HPA coordination states elucidates ion-selective transport, and the relatively lower depth of energy well for the exchange from Li+ hydration to Li+-HPA coordination results in the separation of Li+ from other alkali cations. This work demonstrates a principle for exploring novel ligands to develop alkali cation-selective membranes, expanding the potential applications of ion separation membranes in lithium extraction from aquatic sources.
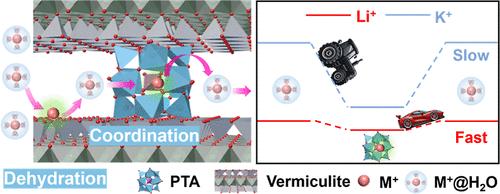
二维通道中的杂多酸配体使锂与一价阳离子分离
从盐湖中提取锂需要具有可定制纳米通道的离子选择膜。然而,由于碱阳离子具有相同的价和相似的离子半径,因此分离碱阳离子仍然是一个主要的挑战。受KcsA K+的K+通道的启发,在调节纳米通道尺寸以控制碱阳离子脱水主导的离子选择性方面取得了重大进展。此外,基于配位化学将冠醚等配体掺入纳米通道中,以提高碱阳离子的选择性;然而,只实现了单/二价阳离子之间的分离。本文设计了一系列杂多酸(HPA)配体来功能化二维(2D)纳米通道,实现了比其他碱阳离子(Li+/K+为16)更高的锂离子选择性,Li+渗透率提高到原始二维膜的4倍。我们发现离子在水合态和离子-HPA配位态之间的切换说明了离子的选择性输运,而从Li+水合态到Li+-HPA配位的相对较低的能量阱深度导致了Li+与其他碱阳离子的分离。这项工作为探索新型配体开发碱阳离子选择性膜提供了一个原理,扩大了离子分离膜在水生锂提取中的潜在应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
文献相关原料
公司名称
产品信息
阿拉丁
MgCl<sub>2</sub>
阿拉丁
hexamethylenetetramine (C<sub>6</sub>H<sub>12</sub>N<sub>4</sub>)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


