Lewis Acid–Base Pairs Constructed via Lattice Regulation for Ultrafast Catalytic Transfer Hydrogenation
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Catalytic transfer hydrogenation (CTH) strongly relies on the synergistic interaction between Lewis acid and Lewis base. Highly active, high-density, and well-dispersed Lewis acid–base pairs (LP) are crucial to achieving efficient CTH catalysis, yet forming such an ideal interface remains challenging. To address this, a novel construction strategy is presented, which leverages the regulation of the layered double hydroxide (LDH) lattice structure to establish an ideal LP interface. Supercritical isopropyl alcohol (SCIP) was employed to selectively remove hydroxyl groups and hydrogen bonds from the NiAl-LDH surface, constructing rich MCUS and Ni-OOH at the LDH interface in a simple, controllable, and environmentally friendly way. The formation process of MCUS and Ni-OOH in SCIP was analyzed using a series of dynamic characterization. Key factors restricting the formation of MCUS and Ni-OOH were identified by comparing results across different precursor preparation methods and temperatures of SCIP treatment. On this basis, the one-pot reaction system was established. Within this system, catalyst preparation and the CTH of ethyl levulinate (EL) to γ-valerolactone (GVL) co-occur. The system simplifies the CTH reaction process and exhibits ultrahigh catalytic efficiency, with a GVL formation rate of 0.780 molGVL·g–1·h–1. Compared to traditional reaction systems and catalysts, the developed one-pot reaction system and catalyst demonstrates significant advantages and exhibit excellent cyclic stability after catalyst stabilization. The combination of the LP interface and the one-pot reaction system enabled environmentally friendly, economical, and efficient biomass-based GVL synthesis.
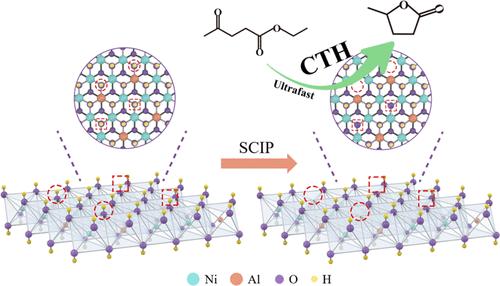
晶格调控构建的路易斯酸碱对用于超快催化转移加氢
催化转移加氢反应(CTH)强烈依赖于路易斯酸和路易斯碱之间的协同作用。高活性、高密度、分散良好的刘易斯酸碱对(LP)是实现高效CTH催化的关键,但形成这样一个理想的界面仍然是一个挑战。为了解决这个问题,提出了一种新的构建策略,该策略利用层状双氢氧化物(LDH)晶格结构的调节来建立理想的LP界面。利用超临界异丙醇(SCIP)选择性去除NiAl-LDH表面的羟基和氢键,以简单、可控、环保的方式在LDH界面构建丰富的MCUS和Ni-OOH。通过一系列的动态表征分析了SCIP中mcu和Ni-OOH的形成过程。通过比较不同前驱体制备方法和SCIP处理温度的结果,确定了制约MCUS和Ni-OOH形成的关键因素。在此基础上,建立了单釜反应体系。在该体系中,催化剂制备和乙酰丙酸乙酯(EL)生成γ-戊内酯(GVL)的CTH同时发生。该体系简化了CTH反应过程,具有超高的催化效率,GVL的生成速率为0.780 molGVL·g-1·h-1。与传统的反应体系和催化剂相比,所开发的一锅反应体系和催化剂具有明显的优势,催化剂稳定化后具有良好的循环稳定性。结合LP界面和一锅反应系统,实现了环保、经济、高效的生物质基GVL合成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
文献相关原料
公司名称
产品信息
阿拉丁
ethyl levulinate (EL)
阿拉丁
isopropyl alcohol (IPA)
阿拉丁
NaOH
阿拉丁
Na2CO3
阿拉丁
urea
阿拉丁
Al(NO3)3·9H2O
阿拉丁
Ni(NO3)2·6H2O
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


