High-Density Iron–Nickel Dual Sites in Carbon Aerogels as Effective Alkaline Water/Seawater Oxidation Electrocatalysts
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Carbon-based nanocomposites with atomically dispersed transition metals have been found to exhibit excellent electrocatalytic activity toward the oxygen evolution reaction (OER). Yet, the low metal loads and severe electrooxidation of carbon greatly limit the activity and stability. Reducing the pyrolysis temperature can weaken the aggregation of metal atoms, and using carbon aerogel as a 3D scaffold can maximize accessible metal sites. Simultaneously, a lower pyrolysis temperature can provide a higher oxygen content for the carbon substrate and enhance resistance against electrooxidation. Herein, carbon aerogels embedded with Fe–Ni dual atom centers (NCA/FeNi-500) are synthesized by controlled pyrolysis at 500 °C of a chitosan hydrogel composite along with FeCl3 and NiCl2. With an atomically dispersed metal loading of 4.35 wt %, NCA/FeNi-500 exhibits a remarkable OER catalytic activity in both alkaline water and simulated alkaline seawater, featuring a low overpotential of only +294 and +306 mV to reach the current density of 10 mA cm–2, respectively, along with excellent long-term stability during overall water splitting, a performance much better than those with commercial RuO2. First-principles calculations show that adjacent NiN4 sites effectively promote the OER kinetics at FeN4 sites by reducing the energy barrier of O–O formation. This is also manifested in alkaline saline water splitting.
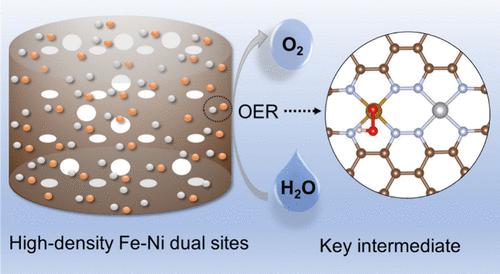
碳气凝胶中的高密度铁镍双位点作为有效的碱性水/海水氧化电催化剂
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


