Mechanochemical Urea Synthesis Using Ammonia–Water and Carbon Dioxide Under Mild Conditions: An Experimental and Theoretical Study
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The production of urea predominantly relies on the energy-intensive Bosch–Meiser process, which operates at temperatures ranging from 150 to 200 °C and pressures of approximately 150 to 250 bar. More sustainable approaches to urea synthesis under milder conditions remain a significant challenge. Herein, we demonstrate that urea can be synthesized via a mechanochemical method using ammonia–water and CO2 under an ambient environment. Without extra catalysts, the ZrO2 texture of the jar and grinding balls has a crucial mechanocatalytic effect on direct urea synthesis. Experimental data coupled with theoretical calculation results indicate that the mechano-induced oxygen vacancies (OV) within the (101) crystal plane of ZrO2 play a pivotal role in urea formation. These vacancies notably reduce the energy barrier for the generation of *NH2 and the subsequent decomposition of NH2COOH, thereby facilitating a more energy-efficient urea synthesis process. This work presents a novel method for synthesizing urea under mild conditions, offering potential cost-effective alternatives to urea production.
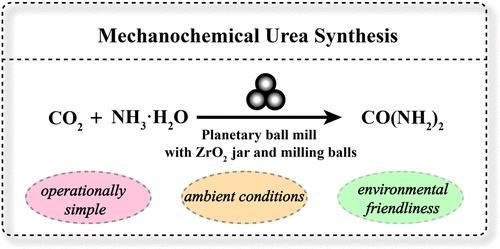
温和条件下氨-水-二氧化碳机械化学合成尿素的实验与理论研究
尿素的生产主要依赖于能源密集型的Bosch-Meiser工艺,该工艺在150至200°C的温度和150至250 bar的压力下运行。在温和条件下更可持续的尿素合成方法仍然是一个重大挑战。在此,我们证明了尿素可以在自然环境下通过氨-水和二氧化碳的机械化学方法合成。在没有额外催化剂的情况下,罐子和磨球的ZrO2织构对直接合成尿素具有重要的机械催化作用。实验数据和理论计算结果表明,ZrO2(101)晶面内的机械诱导氧空位(OV)在尿素生成中起着关键作用。这些空位显著降低了*NH2生成和随后NH2COOH分解的能垒,从而促进了更节能的尿素合成过程。这项工作提出了一种在温和条件下合成尿素的新方法,为尿素生产提供了潜在的经济有效的替代品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
文献相关原料
公司名称
产品信息
阿拉丁
Urea
阿拉丁
Thiosemicarbazide
阿拉丁
Diacetylmonoxime
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


