Development of an FKBP12-recruiting chemical-induced proximity DNA-encoded library and its application to discover an autophagy potentiator
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
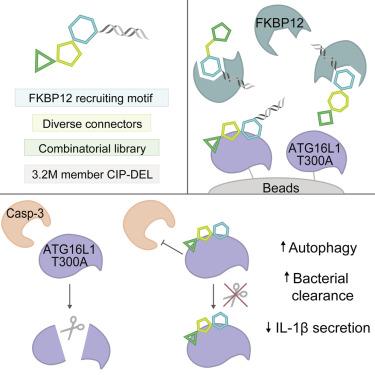
Chemical inducers of proximity (CIPs) are molecules that recruit one protein to another and introduce new functionalities toward modulating protein states and activities. While CIP-mediated recruitment of E3 ligases is widely exploited for the development of degraders, other therapeutic modalities remain underexplored. We describe a non-degrader CIP-DNA-encoded library (CIP-DEL) that recruits FKBP12 to target proteins using non-traditional acyclic structures, with an emphasis on introducing stereochemically diverse and rigid connectors to attach the combinatorial library. We deployed this strategy to modulate ATG16L1 T300A, which confers genetic susceptibility to Crohn’s disease (CD), and identified a compound that stabilizes the variant protein against caspase-3 (Casp3) cleavage in a FKBP12-independent manner. We demonstrate in cellular models that this compound potentiates autophagy, and reverses the xenophagy defects as well as increased cytokine secretion characteristic of ATG16L1 T300A. This study provides a platform to access unexplored chemical space for CIP design to develop therapeutic modalities guided by human genetics.

fkbp12募集化学诱导邻近dna编码文库的建立及其在发现自噬增强剂中的应用
化学接近诱导剂(Chemical inductors of proximity, cip)是一种将一种蛋白质招募到另一种蛋白质并引入新功能来调节蛋白质状态和活性的分子。虽然cip介导的E3连接酶募集被广泛用于降解物的开发,但其他治疗方式仍未得到充分探索。我们描述了一个非降解的cip - dna编码文库(CIP-DEL),它使用非传统的无环结构招募FKBP12来靶向蛋白质,重点是引入立体化学多样性和刚性连接器来连接组合文库。我们采用这种策略来调节ATG16L1 T300A,它赋予克罗恩病(CD)的遗传易感性,并鉴定了一种化合物,该化合物以不依赖于fkbp12的方式稳定变异蛋白,防止Casp3切割。我们在细胞模型中证明,这种化合物增强了自噬,逆转了ATG16L1 T300A的异种吞噬缺陷以及增加的细胞因子分泌特征。这项研究为CIP设计提供了一个未经探索的化学空间,以开发由人类遗传学指导的治疗方式。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


