Evolutionary study and structural basis of proton sensing by Mus GPR4 and Xenopus GPR4
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
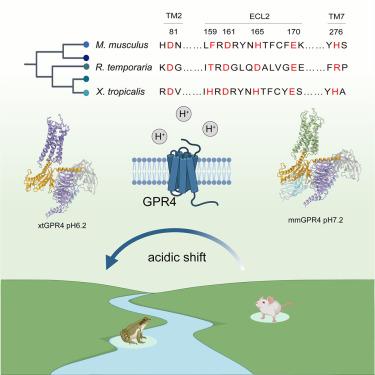
Animals have evolved pH-sensing membrane receptors, such as G-protein-coupled receptor 4 (GPR4), to monitor pH changes related to their physiology and generate adaptive reactions. However, the evolutionary trajectory and structural mechanism of proton sensing by GPR4 remain unresolved. Here, we observed a positive correlation between the optimal pH of GPR4 activity and the blood pH range across different species. By solving 7-cryoelectron microscopy (cryo-EM) structures of Xenopus tropicalis GPR4 (xtGPR4) and Mus musculus GPR4 (mmGPR4) under varying pH conditions, we identified that protonation of HECL2-45.47 and H7.36 enabled polar network establishment and tighter association between the extracellular loop 2 (ECL2) and 7 transmembrane (7TM) domain, as well as a conserved propagating path, which are common mechanisms underlying protonation-induced GPR4 activation across different species. Moreover, protonation of distinct extracellular HECL2-45.41 contributed to the more acidic optimal pH range of xtGPR4. Overall, our study revealed common and distinct mechanisms of proton sensing by GPR4, from a structural, functional, and evolutionary perspective.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


