Biocatalytic Efficient and Enantiocomplementary Synthesis of 3-Hydroxy-3-hydroxymethyloxindoles by Combining Halohydrin Dehalogenase and Epoxide Hydrolase
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
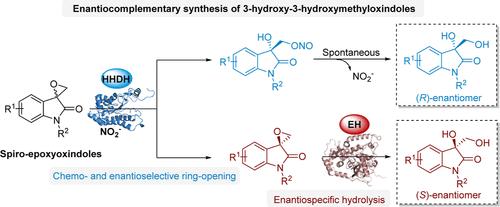
Enantiopure 3-hydroxyoxindoles are one class of basic functional molecules that hold particular interest in medicinal chemistry and drug discovery due to their diverse pharmacological properties. While many chemical methods have been developed for producing these molecules, there remains a continuous demand for more efficient and greener approaches. Herein, we present a novel dual-enzyme biocatalytic platform for the enantiocomplementary synthesis of chiral 3-hydroxy-3-hydroxymethyloxindoles, compounds that have not previously been synthesized stereoselectively. This biocatalytic platform involves the halohydrin dehalogenase-catalyzed kinetic resolution of racemic spiro-epoxyoxindoles with nitrite, paired with the epoxide hydrolase-catalyzed enantiospecific hydrolysis of the residual enantiopure spiro-epoxyoxindoles. Both enzymatic processes demonstrate high catalytic selectivity and efficiency, enabling the preparative synthesis of various (R)- and (S)-3-hydroxy-3-hydroxymethyloxindoles with high yields (up to 50%) and optical purities (up to >99% ee). In addition, useful transformations of the chiral products were conducted to further showcase the scalability and applicability of the biocatalytic platform.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


