Tunable Bicontinuous Macroporous Cell Culture Scaffolds via Kinetically Controlled Phase Separation
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
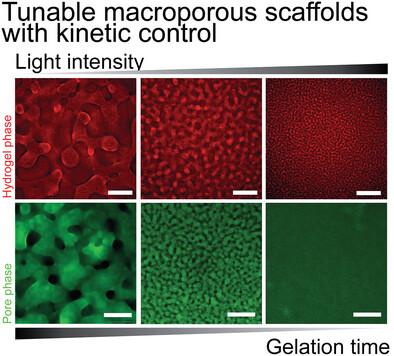
3D scaffolds enable biological investigations with a more natural cell conformation. However, the porosity of synthetic hydrogels is often limited to the nanometer scale, which confines the movement of 3D encapsulated cells and restricts dynamic cell processes. Precise control of hydrogel porosity across length scales remains a challenge and the development of porous materials that allow cell infiltration, spreading, and migration in a manner more similar to natural ECM environments is desirable. Here, a straightforward and reliable method is presented for generating kinetically-controlled macroporous biomaterials using liquid–liquid phase separation between poly(ethylene glycol) (PEG) and dextran. Photopolymerization-induced phase separation resulted in macroporous hydrogels with tunable pore size. Varying light intensity and hydrogel composition controlled polymerization kinetics, time to percolation, and complete gelation, which defined the average pore diameter (Ø = 1–200 µm) and final gel stiffness of the formed hydrogels. Critically, for biological applications, macroporous hydrogels are prepared from aqueous polymer solutions at physiological pH and temperature using visible light, allowing for direct cell encapsulation. Human dermal fibroblasts in a range of macroporous gels are encapsulated with different pore sizes. Porosity improved cell spreading with respect to bulk gels and allowed migration in the porous biomaterials.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


