A Hydrazine‒Water Galvanic Cell-Inspired Self-Powered High-Rate Hydrogen Production via Overall Hydrazine Electrosplitting
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
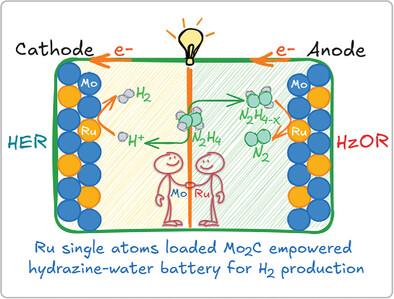
Exploring advanced electrolysis techniques for attaining scene-adaptive and on-site green H2 production is an imperative matter of utmost practical significance but grand challenge remains. Herein, drawn inspiration from a spontaneous hydrazine‒H2O galvanic cell configured on a low-valence Ru single atoms-loaded Mo2C electrode (RuSA/v-Mo2C), an alternative H2 energy solution utilizing self-powered electrochemical hydrazine splitting (N2H4 → 2H2 + N2) instead of the stereotyped electricity-consumed water splitting for green H2 production is proposed. This solution highlights a pH-decoupled hydrazine‒H2O primary battery with notable open-circuit voltage of 1.37 V and energy density up to 358 Wh gN2H4−1, which powerfully propels an alkaline hydrazine splitting cell, leading to bilateral H2 harvest with a remarkable rate of 18 mol h−1 m−2, i.e., 403.2 L h−1 m−2, setting a new record for the self-sustaining electricity-powered H2 production systems. The success of RuSA/v-Mo2C for this solution is further decoded by tandem theoretical and in situ spectroscopic studies, cross-verifying a Ru‒Mo dual-site synergy in streamlining the overall energy barriers, thereby enhancing the kinetics of electrode reactions. This pioneering work, showcasing electrochemical H2 production free from both external energy and feedstock inputs, opens up a new horizon on way of the ultimate H2 energy solution.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


