Gliomagenesis mimics an injury response orchestrated by neural crest-like cells
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
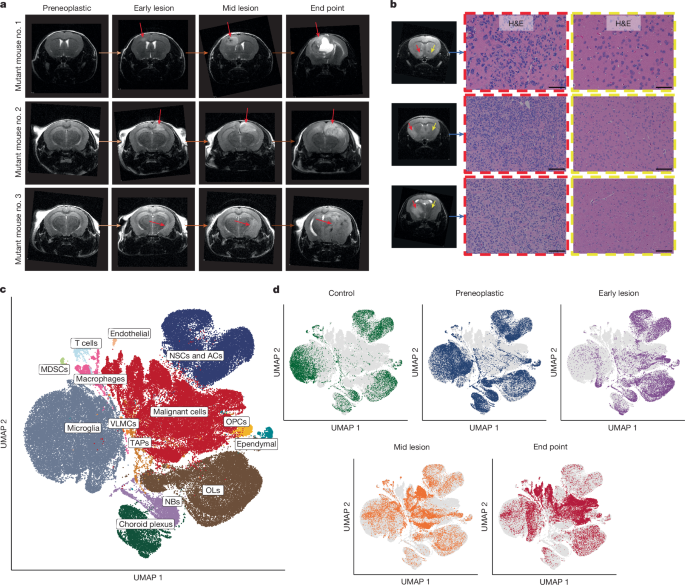
Glioblastoma is an incurable brain malignancy. By the time of clinical diagnosis, these tumours exhibit a degree of genetic and cellular heterogeneity that provides few clues to the mechanisms that initiate and drive gliomagenesis1,2. Here, to explore the early steps in gliomagenesis, we utilized conditional gene deletion and lineage tracing in tumour mouse models, coupled with serial magnetic resonance imaging, to initiate and then closely track tumour formation. We isolated labelled and unlabelled cells at multiple stages—before the first visible abnormality, at the time of the first visible lesion, and then through the stages of tumour growth—and subjected cells of each stage to single-cell profiling. We identify a malignant cell state with a neural crest-like gene expression signature that is highly abundant in the early stages, but relatively diminished in the late stage of tumour growth. Genomic analysis based on the presence of copy number alterations suggests that these neural crest-like states exist as part of a heterogeneous clonal hierarchy that evolves with tumour growth. By exploring the injury response in wounded normal mouse brains, we identify cells with a similar signature that emerge following injury and then disappear over time, suggesting that activation of an injury response program occurs during tumorigenesis. Indeed, our experiments reveal a non-malignant injury-like microenvironment that is initiated in the brain following oncogene activation in cerebral precursor cells. Collectively, our findings provide insight into the early stages of glioblastoma, identifying a unique cell state and an injury response program tied to early tumour formation. These findings have implications for glioblastoma therapies and raise new possibilities for early diagnosis and prevention of disease. A study using glioblastoma mouse models, serial magnetic resonance imaging and single-cell profiling details changes in the identity and balance of cellular states from initiation of tumorigenesis to the end point.

神经胶质瘤形成模拟了神经嵴样细胞的损伤反应
胶质母细胞瘤是一种无法治愈的脑部恶性肿瘤。到临床诊断时,这些肿瘤表现出一定程度的遗传和细胞异质性,这为启动和驱动胶质瘤形成的机制提供了很少的线索1,2。在这里,为了探索胶质瘤形成的早期步骤,我们在肿瘤小鼠模型中使用条件基因缺失和谱系追踪,结合序列磁共振成像,启动并密切跟踪肿瘤的形成。我们在多个阶段分离标记和未标记的细胞-在第一个可见异常之前,在第一个可见病变时,然后在肿瘤生长的各个阶段-并对每个阶段的细胞进行单细胞谱分析。我们发现了一种具有神经嵴样基因表达特征的恶性细胞状态,这种基因表达特征在肿瘤生长的早期阶段高度丰富,但在肿瘤生长的晚期相对减少。基于拷贝数改变的基因组分析表明,这些神经嵴样状态是随着肿瘤生长而进化的异质克隆层次结构的一部分。通过探索受伤的正常小鼠大脑中的损伤反应,我们发现了具有类似特征的细胞,这些细胞在损伤后出现,然后随着时间的推移消失,这表明在肿瘤发生过程中发生了损伤反应程序的激活。事实上,我们的实验揭示了一种非恶性损伤样微环境,它是在大脑前体细胞中癌基因激活后在大脑中启动的。总的来说,我们的发现为胶质母细胞瘤的早期阶段提供了见解,确定了与早期肿瘤形成相关的独特细胞状态和损伤反应程序。这些发现对胶质母细胞瘤的治疗具有重要意义,并为疾病的早期诊断和预防提供了新的可能性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


