Empagliflozin in nondiabetic individuals with calcium and uric acid kidney stones: a randomized phase 2 trial
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Efficacy of sodium-glucose cotransporter 2 inhibitors for kidney stone prevention in nondiabetic patients is unknown. In a double-blind, placebo-controlled, single-center, crossover phase 2 trial, 53 adults (≥18 and <75 years) with calcium (n = 28) or uric acid (UA; n = 25) kidney stones (at least one previous kidney stone event) without diabetes (HbA1c < 6.5%, no diabetes treatment) were randomized to once daily empagliflozin 25 mg followed by placebo or reverse (2 weeks per treatment). Randomization and analysis were performed separately for both stone types. Primary analyses were conducted in the per protocol set. Primary outcomes were urine relative supersaturation ratios (RSRs) for calcium oxalate (CaOx), calcium phosphate (CaP) and UA—validated surrogates for stone recurrence. Prespecified RSR reductions (≥15%) were met in both groups of stone formers. In patients with calcium stones, empagliflozin reduced RSR CaP (relative difference to placebo, −36%; 95% confidence interval, −48% to −21%; P < 0.001), but not RSRs CaOx and UA. In patients with UA stones, empagliflozin reduced RSR UA (−30%; 95% confidence interval, −44% to −12%; P = 0.002) but not RSRs CaOx and CaP. No serious or prespecified adverse events occurred. Thus, empagliflozin substantially reduced RSRs in nondiabetic adults with calcium and UA kidney stones. ClinicalTrials.gov registration: NCT04911660 . As part of the SWEETSTONE trial, the authors report the ability of the sodium-glucose cotransporter 2 inhibitor empagliflozin to reduce the likelihood of kidney stone formation in individuals without diabetes.
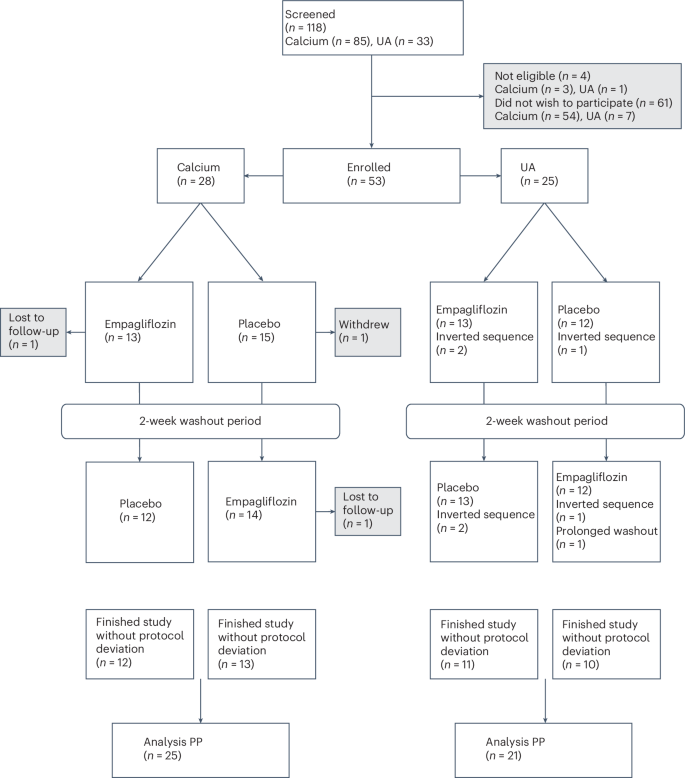

恩格列净治疗患有钙和尿酸性肾结石的非糖尿病患者:一项随机2期试验
钠-葡萄糖共转运蛋白2抑制剂预防非糖尿病患者肾结石的疗效尚不清楚。在一项双盲、安慰剂对照、单中心、交叉2期试验中,53名成年人(≥18岁和75岁)服用钙(n = 28)或尿酸(UA;n = 25)肾结石(至少有一次肾结石事件),无糖尿病(HbA1c < 6.5%,未接受糖尿病治疗),随机分为每日1次恩帕列净25mg,随后服用安慰剂或逆转(每次治疗2周)。对两种结石类型分别进行随机化和分析。对每个方案集进行初步分析。主要结局是草酸钙(CaOx)、磷酸钙(CaP)和ua验证的结石复发替代物的尿液相对过饱和度(rrs)。两组结石患者均达到了预先规定的RSR降低(≥15%)。在钙结石患者中,恩格列净降低了RSR CaP(与安慰剂的相对差异为- 36%;95%置信区间为- 48% ~ - 21%;P < 0.001),但不包括RSRs CaOx和UA。在UA结石患者中,恩格列净降低RSR UA (- 30%;95%置信区间为- 44% ~ - 12%;P = 0.002),但未出现RSRs CaOx和CaP。未发生严重或预先规定的不良事件。因此,恩格列净显著降低了患有钙和UA肾结石的非糖尿病成人的RSRs。ClinicalTrials.gov注册:NCT04911660。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


