Design, synthesis, and biological evaluation of a potent and orally bioavailable FGFRs inhibitor for fibrotic treatment
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Organ fibrosis, such as lung fibrosis and liver fibrosis, is a progressive and fatal disease. Fibroblast growth factor receptors (FGFRs) play an important role in the development and progression of fibrosis. Through scaffold hopping, bioisosteric replacement design, and structure-activity relationship optimization, we developed a series of highly potent FGFRs inhibitors, and the indazole-containing candidate compound A16 showed potent kinase activity comparable to that of AZD4547. In addition, A16 effectively suppressed the activation of lung fibroblasts and hepatic stellate cells (HSCs) induced by TGF-β1, leading to a reduction in collagen deposition. Notably, A16 exhibited potent anti-fibrotic effects through the inhibition of the FGFR pathway in vitro. Compound A16 also showed reasonable pharmacokinetic properties (F = 21.84 %) and favorable cardiac safety (hERG IC50 > 20 μM). Moreover, in models of pulmonary fibrosis, A16 ameliorated (in the prevention model) and reversed (in the treatment model) bleomycin-induced lung fibrosis, as well as mitigated inflammatory immune response in the lung. Furthermore, in the CCl4-induced liver fibrosis model, when A16 was administrated orally at a dose of 30 mg/kg/day for 3 weeks, it effectively improved liver function, restored damaged liver structures, and reduced collagen deposition. Taken together, these results suggest that A16 could be a potential drug candidate for the treatment of organ fibrosis.
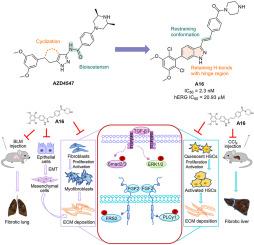

一种有效的口服FGFRs抑制剂的设计、合成和生物学评价
器官纤维化,如肺纤维化和肝纤维化,是一种进行性和致命性疾病。成纤维细胞生长因子受体(FGFRs)在纤维化的发生和发展中起着重要作用。通过支架跳跃、生物等构替代设计和构效关系优化,我们开发了一系列高效的FGFRs抑制剂,含吲达唑的候选化合物A16显示出与AZD4547相当的强效激酶活性。此外,A16有效抑制TGF-β1诱导的肺成纤维细胞和肝星状细胞(HSCs)的活化,导致胶原沉积减少。值得注意的是,A16在体外通过抑制FGFR通路表现出强大的抗纤维化作用。化合物A16也具有合理的药动学性质(F = 21.84%)和良好的心脏安全性(hERG IC50 >;20μM)。此外,在肺纤维化模型中,A16改善(预防模型)和逆转(治疗模型)博莱霉素诱导的肺纤维化,并减轻肺炎症免疫反应。此外,在ccl4诱导的肝纤维化模型中,A16以30 mg/kg/天的剂量口服3周后,可有效改善肝功能,恢复受损的肝脏结构,减少胶原沉积。综上所述,这些结果表明A16可能是治疗器官纤维化的潜在候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


