KIF1C activates and extends dynein movement through the FHF cargo adapter
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
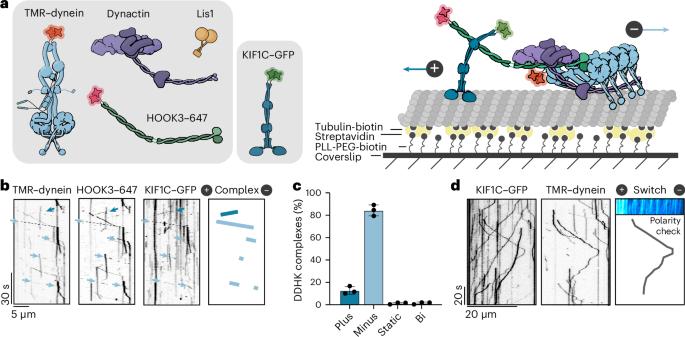
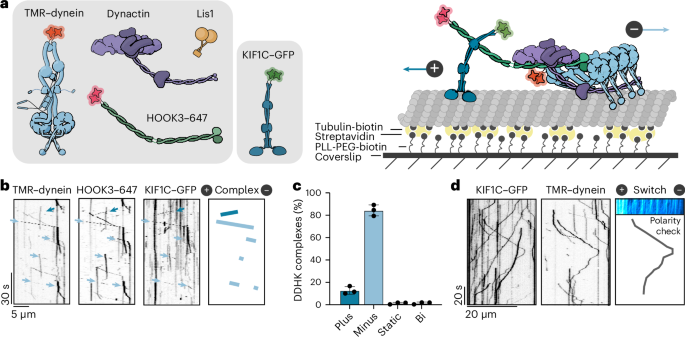
Cellular cargos move bidirectionally on microtubules by recruiting opposite polarity motors dynein and kinesin. These motors show codependence, where one requires the activity of the other, although the mechanism is unknown. Here we show that kinesin-3 KIF1C acts as both an activator and a processivity factor for dynein, using in vitro reconstitutions of human proteins. Activation requires only a fragment of the KIF1C nonmotor stalk binding the cargo adapter HOOK3. The interaction site is separate from the constitutive factors FTS and FHIP, which link HOOK3 to small G-proteins on cargos. We provide a structural model for the autoinhibited FTS–HOOK3–FHIP1B (an FHF complex) and explain how KIF1C relieves it. Collectively, we explain codependency by revealing how mutual activation of dynein and kinesin occurs through their shared adapter. Many adapters bind both dynein and kinesins, suggesting this mechanism could be generalized to other bidirectional complexes. Here the authors show that codependence of dynein and kinesin KIF1C occurs through binding of the FTS–HOOK3–FHIP1B cargo adapter. Binding of KIF1C releases the HOOK3 autoinhibited folded conformation allowing dynein to bind the adapter. In this cocomplex, KIF1C further acts as a processivity factor for dynein.
KIF1C通过FHF货运适配器激活并扩展动力蛋白运动
细胞货物通过吸收极性相反的马达——动力蛋白和运动蛋白,在微管上双向移动。这些马达表现出相互依赖,其中一个需要另一个的活动,尽管机制尚不清楚。在这里,我们展示了KIF1C既作为动力蛋白的激活剂,又作为动力蛋白的加工因子,使用人类蛋白的体外重组。激活只需要KIF1C非运动梗的一个片段结合货物适配器HOOK3。相互作用位点与构成因子FTS和FHIP分离,后者将HOOK3与货物上的小g蛋白连接起来。我们提供了一个自抑制ffs - hook3 - fhip1b(一种FHF复合物)的结构模型,并解释了KIF1C如何缓解它。总的来说,我们通过揭示动力蛋白和动力蛋白如何通过它们的共享适配器相互激活来解释相互依赖。许多适配器结合动力蛋白和动力蛋白,表明这种机制可以推广到其他双向复合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


