Mapping the metagenomic diversity of the multi-kingdom glacier-fed stream microbiome
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
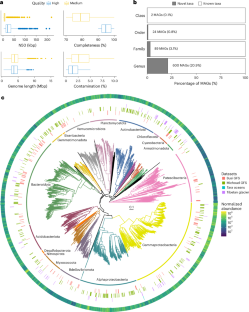
Glacier-fed streams (GFS) feature among Earth’s most extreme aquatic ecosystems marked by pronounced oligotrophy and environmental fluctuations. Microorganisms mainly organize in biofilms within them, but how they cope with such conditions is unknown. Here, leveraging 156 metagenomes from the Vanishing Glaciers project obtained from sediment samples in GFS from 9 mountains ranges, we report thousands of metagenome-assembled genomes (MAGs) encompassing prokaryotes, algae, fungi and viruses, that shed light on biotic interactions within glacier-fed stream biofilms. A total of 2,855 bacterial MAGs were characterized by diverse strategies to exploit inorganic and organic energy sources, in part via functional redundancy and mixotrophy. We show that biofilms probably become more complex and switch from chemoautotrophy to heterotrophy as algal biomass increases in GFS owing to glacier shrinkage. Our MAG compendium sheds light on the success of microbial life in GFS and provides a resource for future research on a microbiome potentially impacted by climate change. Thousands of metagenome-assembled genomes from the Vanishing Glaciers project showcase the interactions between prokaryotes, algae, fungi and viruses in glacier-fed stream environments.

绘制多王国冰川喂养溪流微生物组的宏基因组多样性
冰川补给河流(GFS)是地球上最极端的水生生态系统之一,其特征是明显的少营养化和环境波动。微生物主要在它们内部的生物膜中组织,但它们如何应对这种条件尚不清楚。在这里,利用从9个山脉的GFS沉积物样本中获得的冰川消失项目的156个宏基因组,我们报告了数千个包含原核生物、藻类、真菌和病毒的宏基因组组装基因组(MAGs),这些基因组揭示了冰川补给流生物膜内的生物相互作用。共有2,855个细菌mag具有不同的利用无机和有机能源的策略,部分是通过功能冗余和混合营养。我们发现,由于冰川萎缩,GFS中藻类生物量增加,生物膜可能变得更加复杂,并从化学自养转变为异养。我们的MAG概要揭示了GFS中微生物生命的成功,并为未来研究可能受气候变化影响的微生物组提供了资源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


