Cerebellar Bergmann glia integrate noxious information and modulate nocifensive behaviors
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
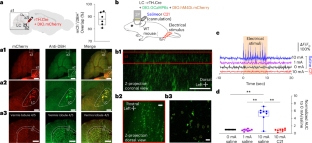
The cerebellum is activated by noxious stimuli and pathological pain but its role in noxious information processing remains unknown. Here, we show that in mice, cutaneous noxious electrical stimuli induced noradrenaline (NA) release from locus coeruleus (LC) terminals in the cerebellar cortex. Bergmann glia (BG) accumulated these LC–NA signals by increasing intracellular calcium in an integrative manner (‘flares’). BG flares were also elicited in response to an intraplantar capsaicin injection. Chemogenetic inactivation of LC terminals or BG in the cerebellar cortex or BG-specific knockdown of α1-adrenergic receptors suppressed BG flares, reduced nocifensive licking and had analgesic effects in nerve injury-induced chronic neuropathic pain. Moreover, chemogenetic activation of BG or an intraplantar capsaicin injection reduced Purkinje cell firing, which may disinhibit the output activity of the deep cerebellar nuclei. These results suggest a role for BG in computing noxious information from the LC and in modulating pain-related behaviors by regulating cerebellar output. This study shows that cerebellar Bergmann glia process noxious stimuli by integrating signals from the locus coeruleus. This mechanism modulates pain-related behaviors. These findings provide insight into cerebellar involvement in pain processing.

小脑伯格曼神经胶质整合有害信息并调节有害行为
小脑被有害刺激和病理性疼痛激活,但其在有害信息处理中的作用尚不清楚。在小鼠中,皮肤有害电刺激诱导小脑皮质蓝斑(LC)末端释放去甲肾上腺素(NA)。伯格曼胶质细胞(BG)通过综合方式增加细胞内钙来积累这些LC-NA信号(“耀斑”)。足底辣椒素注射也引起了BG耀斑。小脑皮质LC末端或BG的化学发生失活或α1-肾上腺素能受体的BG特异性敲低可抑制BG耀斑,减少有害舔舐,并对神经损伤引起的慢性神经性疼痛具有镇痛作用。此外,化学发生激活BG或足底注射辣椒素可减少浦肯野细胞放电,这可能解除了对小脑深部核输出活性的抑制。这些结果表明BG在计算来自LC的有害信息和通过调节小脑输出调节疼痛相关行为方面发挥作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


