A Review of Electrochemistry of osmium(II)-polypyridines and supporting DFT studies
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
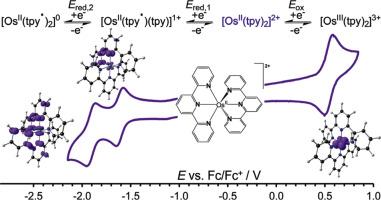
This work presents a critical review of the electrochemistry of osmium(II)-polypyridines, supported by Density Functional Theory (DFT) studies, focusing on phenanthroline, bipyridine, terpyridine, and their substituted derivatives. The results demonstrate that osmium(II)-bipyridines, osmium(II)-phenanthrolines, and osmium(II)-terpyridines exhibit similar redox behavior. The reversible one-electron oxidation of these d6 osmium(II)-polypyridines leads to the formation of d5 osmium(III)-polypyridines, which maintain a similar molecular structure. The one-electron reduction of these osmium(II)-polypyridines produces a reduced molecule with a d6 osmium(II) center, accompanied by one ligand radical (containing one unpaired electron) and one neutral ligand (for terpyridine) or two neutral ligands (for phenanthroline or bipyridine). The electronic structures of DFT-optimized osmium(III)-polypyridines, osmium(II)-polypyridines, and their reduced forms support the experimental assignment of the observed redox processes. Additionally, the oxidation potentials of osmium(II)-polypyridines are determined to be directly related to the highest occupied molecular orbital (HOMO) energies of the DFT-optimized osmium(II)-polypyridines making it possible to predict redox potentials of related systems. In conclusion, the limitations of the published work are critically assessed, and potential future perspectives are thoughtfully explored.
锇(II)-聚吡啶的电化学及其支持的DFT研究综述
本文在密度泛函理论(DFT)的支持下,对锇(II)-多吡啶的电化学进行了综述,重点研究了菲罗啉、联吡啶、三吡啶及其取代衍生物。结果表明,锇(II)-联吡啶、锇(II)-菲罗啉和锇(II)-三吡啶表现出相似的氧化还原行为。这些d6锇(II)-多吡啶的可逆单电子氧化导致d5锇(III)-多吡啶的形成,它们保持相似的分子结构。这些锇(II)-多吡啶的单电子还原产生具有d6锇(II)中心的还原分子,伴随着一个配体自由基(含有一个未配对电子)和一个中性配体(对于三吡啶)或两个中性配体(对于菲罗啉或联吡啶)。dft优化的锇(III)-聚吡啶、锇(II)-聚吡啶及其还原形式的电子结构支持观察到的氧化还原过程的实验指派。此外,确定了锇(II)-多吡啶的氧化电位与dft优化的锇(II)-多吡啶的最高占据分子轨道(HOMO)能直接相关,从而可以预测相关体系的氧化还原电位。总之,对已发表的工作的局限性进行了批判性评估,并对潜在的未来前景进行了深思熟虑的探讨。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


