Ruthenium–Hydride Complexes Facilitated Sustainable Synthesis of Isoxazolones via Acceptorless Dehydrogenative Annulation of Alcohols
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
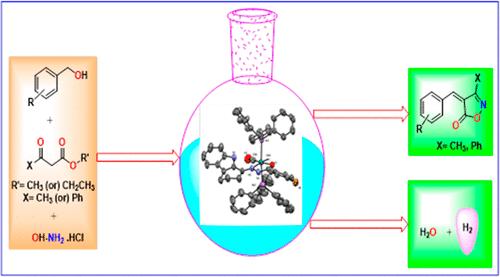
A streamlined strategy for the one-pot synthesis of isoxazolone analogues has been developed through an acceptorless dehydrogenative annulation (ADA) pathway by employing new Ru(II) hydride complexes as effective catalysts. New Ru(II) complexes (C1–C3) tailored with N̂O chelating carbazolone benzhydrazone ligands were synthesized and their formation was confirmed using analytical and spectral techniques including FT-IR and NMR. The structural configuration of the complexes featuring an octahedral geometry around the Ru(II) ion was precisely determined by single-crystal X-ray diffraction analysis. Further, the catalytic efficacy of the titled complexes has been established for the facile and productive synthesis of isoxazolone derivatives from a diverse range of benzyl alcohols, methyl acetoacetate/ethyl benzoylacetate and hydroxylamine hydrochloride, generating excellent yields of up to 93% under well-suited mild conditions with 1 mol % of catalyst loading. A sequence of time-dependent control experiments unveiled the ADA route, indicating the initial generation of an 4-methoxy benzaldehyde intermediate followed by 3-phenylisoxazol-5(4H)-one, accompanied by the release of water and hydrogen as byproducts. Gram-scale synthesis of the compound indicates the industrial relevance of our synthetic strategy. A short synthesis of medicinally active androgen antagonist illustrates the utility of the present protocol.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


