Design and Regulation of Anthraquinone’s Electrochemical Properties in Aqueous Zinc-Ion Batteries via Benzothiadiazole and Its Dinitro Derivatives
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
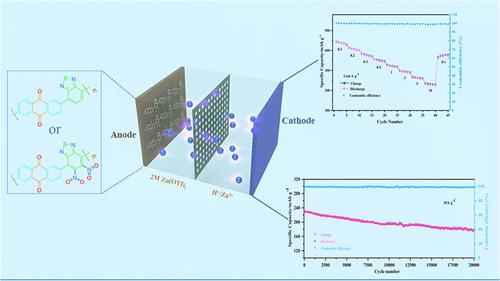
Organic cathode materials are widely considered as highly promising for aqueous zinc-ion batteries (AZIBs) due to their tunable properties, low cost, and ease of processing and synthesis. Benzothiadiazoles have demonstrated significant potential as organic electrode materials in AZIBs, owing to their strong electron-accepting capabilities and the presence of multiple reversible redox sites in anthraquinone. In this study, we designed a polymer, poly(2-methyl-6-(7-methyl-5,6-dinitrobenzo[c][1,2,5]thiadiazol-4-yl)anthracene-9,10-dione) (PBDQ), with multielectron transfer capability through a copolymerization approach. Additionally, we synthesized another polymer, poly2,6-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)anthracene-9,10-dione(PBDQ-N), by introducing two electron-withdrawing nitro groups on the aromatic ring of benzothiadiazole. The introduction of nitro groups, with their unique electronic properties, enhances electron delocalization and increases the number of electrochemically active sites, thereby promoting faster zinc-ion insertion/extraction reactions. Experimental results show that both PBDQ and PBDQ-N exhibit excellent electrochemical properties due to the abundance of active sites and extended π-conjugation. Among them, PBDQ-N demonstrates outstanding performance, including an ultrahigh specific capacity of 446.2 mAh g–1 at 0.1 A g–1 and excellent cycle life exceeding 20,000 cycles at 10 A g–1. Moreover, the lower lowest-unoccupied molecular orbital (LUMO) energy level and improved conductivity of PBDQ-N provide a fast electron transfer rate, resulting in a higher Zn2+ diffusion coefficient (3.47 × 10–11–2.6 × 10–8 cm2 s–1) and exceptional rate performance (234.6 mAh g–1 at 10 A g–1). Theoretical calculations and ex situ characterizations confirm that C═O, C═N, and N═O groups all participate as active sites in Zn2+ storage. This work highlights how molecular design and the introduction of functional groups, such as nitro groups, can effectively regulate the electrochemical properties of organic polymers in AZIBs. It also demonstrates the impact of these strategies on the electrochemical performances of these materials when they are used as cathodes in aqueous zinc-ion batteries.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


