Spontaneous Catalytic Paal–Knorr Reactions for N-Substituted Pyrrole Synthesis by Microdroplet Chemistry
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
It is well recognized in recent studies that water molecules at the gas–liquid interface of microdroplets spontaneously dissociate into hydronium and hydroxide and form superacids/superbases and reactive species (hydrated electrons, hydroxide radicals, hydrogen peroxide, water radical cations/anions, etc.) due to the 109 V m–1 electric field. In contrast to extensive interest in spontaneous redox reactions by the reactive species in microdroplet studies, limited attention has been attracted by gram-scale organic synthesis catalyzed by the spontaneously formed superacids/superbases. This study demonstrates spontaneous organic catalysis of the superacids for Paal–Knorr reactions, one of the most classic pathways to construct N-substituted pyrroles with biologically and pharmaceutically important roles. Paal–Knorr reactions can proceed well with no external catalysts in isopropanol microdroplets within 10 min at room temperature. Sixteen N-substituted pyrroles were synthesized using the microdroplet method with 83–99% yields, several orders of magnitude reaction acceleration (a typical rate acceleration factor of 1.18 × 103 based on the ratio of the rate constants), and a scale-up rate of 5.50 g h–1. By avoiding external catalysts, thermal irradiation, long reaction times, and problematic solvents required by conventional Paal–Knorr methods, the microdroplet method was a green, efficient, and attractive alternative for the construction of pyrroles and their derivatives.
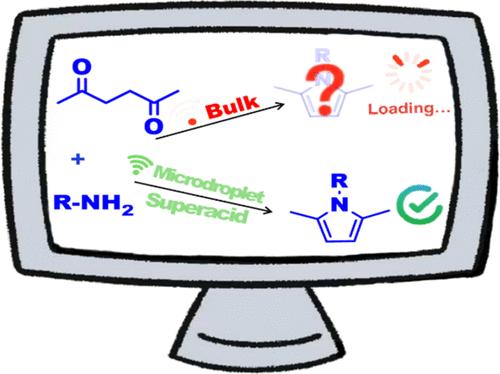
微滴化学合成n -取代吡咯的自发催化Paal-Knorr反应
近年来的研究充分认识到,在109 V m-1的电场作用下,微滴气液界面处的水分子自发解离成水合氢离子和氢氧化物,形成超强酸/超碱和活性物质(水合电子、氢氧自由基、过氧化氢、水自由基阳离子/阴离子等)。与对微滴中活性物质自发氧化还原反应的广泛兴趣相比,对自发形成的超强酸/超碱催化的克级有机合成的关注有限。本研究证明了超强酸对Paal-Knorr反应的自发有机催化作用,这是构建具有重要生物学和药学作用的n -取代吡咯的最经典途径之一。室温条件下,异丙醇微滴中Paal-Knorr反应在无外界催化剂的情况下可在10min内进行。采用微滴法合成了16个n取代吡咯,产率为83 ~ 99%,反应速度加快了几个数量级(基于速率常数之比的典型速率加速因子为1.18 × 103),放大速率为5.50 g h-1。微滴法避免了常规Paal-Knorr法所需要的外部催化剂、热辐射、长反应时间和有问题的溶剂,是一种绿色、高效、有吸引力的构建吡咯及其衍生物的替代方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


