An oxidation-reduction-triggered thiamine disulfide-based prodrug of 10-hydroxycamptothecin for selective tumor cell locking and therapeutic delivery
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Chemotherapy, a primary method of cancer treatment, has been limited in clinical application due to its lack of specificity and tumor multidrug resistance, resulting in numerous undesired side effects. Herein, a small molecule conjugate, TDK-HCPT, was designed and synthesized, which could target tumor cells and prolong the retention of chemotherapy agents within tumor cells. Moreover, a similarly designed control system, TDK-Nap, has been developed as well to enable cancer cell imaging. Two design elements are incorporated into TDK-HCPT: the thiamine disulfide (TDS) and the thioketal subunit (tk). TDS can be reduced in the high glutathione (GSH) conditions within cancer cell to form thiazolium salt, and the resulting enhanced positive charge and lipophobicity make the system difficult to be pumped out of tumor cells, thereby effectively “locking” the chemotherapy drug HCPT inside the tumor cells. Additionally, the tk subunit serves as a ROS trigger, within the tumor cells, the “locked” HCPT were then released and activated by the high ROS conditions, optimizing its targeted potential. This allows TDK-HCPT to serve as a redox-liable molecular platform that targets cancer cells selectively which decreases cancer cell migration, retards tumor growth, and lowers tumorigenesis rates as evidenced by a combination of in vitro and in vivo studies. To the best of our knowledge, this is the first time a cancer cell “lock in” has been shown to prevent tumorigenesis in an animal model.

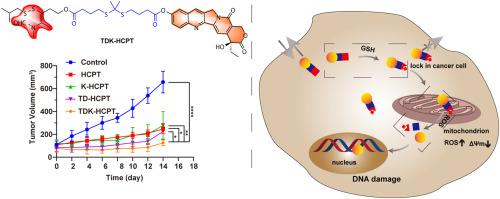
氧化还原触发的基于硫胺素二硫化物的10-羟基喜树碱前药选择性肿瘤细胞锁定和治疗递送
化疗作为癌症治疗的主要方法,由于其缺乏特异性和肿瘤多药耐药,导致许多不良副作用,在临床应用中受到限制。本文设计并合成了一种靶向肿瘤细胞并延长化疗药物在肿瘤细胞内滞留时间的小分子偶联物TDK-HCPT。此外,一个类似设计的控制系统,TDK-Nap,也已开发,使癌细胞成像。TDK-HCPT包含两个设计元素:硫胺素二硫化(TDS)和硫酮亚基(tk)。在癌细胞内高谷胱甘肽(GSH)条件下,TDS可被还原形成噻唑盐,由此增强的正电荷和疏脂性使该系统难以被泵出肿瘤细胞,从而有效地将化疗药物HCPT“锁定”在肿瘤细胞内。此外,tk亚基作为ROS触发器,在肿瘤细胞内,“锁定”的HCPT随后在高ROS条件下被释放和激活,优化其靶向潜力。这使得TDK-HCPT作为一种氧化还原易感的分子平台,选择性地靶向癌细胞,减少癌细胞迁移,延缓肿瘤生长,降低肿瘤发生率,这已被体外和体内研究证明。据我们所知,这是第一次在动物模型中证明癌细胞“锁定”可以防止肿瘤发生。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


