Air stability enhancement and mechanism of Mg-Ca-hydride-based hydrolysis materials
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
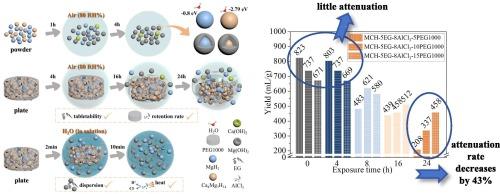
Mg-Ca-hydride-based materials are ideal portable H2 source owing to excellent hydrolysis properties. However, when exposed to air, there is an explosion risk and serious hydrolysis property degradation due to the reaction with steam in air. In this work, by adding 5 wt% expanded graphite, 8 wt% AlCl3 and 5 wt% polyethylene glycol 1000, the H2 yield of a 30 wt% Ca-Mg alloy hydride plate after 4 h air exposure (80 RH%, 25 °C) is increased to 2.3 times that of the original sample without any attenuation. Furthermore, when 15 wt% polyethylene glycol 1000 was added, the attenuation rate after 24 h air exposure is decreased by 43 %. The characterization results show that Ca4Mg3H14 is more easily hydrolyzed than MgH2. First-principles calculations demonstrate that Ca4Mg3H14 interacts with H2O molecules more strongly, owing to larger adsorption energy to H2O. By adding appropriate amounts of expanded graphite, AlCl3 and polyethylene glycol 1000, combined with pressing technology, the direct contact between the material and air can be delayed. Simultaneously, the hydrolysis performance of the material itself can be improved contributed to destruction of the Mg(OH)2 passivation layer by expanded graphite and AlCl3, thereby jointly enhancing the long-term storage performance. This work may advance the engineering application of Mg-Ca-based hydrolysis materials.
镁钙氢化物基水解材料的空气稳定性增强及机理研究
镁钙氢化物基材料具有优异的水解性能,是理想的便携式氢源。然而,当暴露在空气中时,由于与空气中的蒸汽反应,存在爆炸危险和严重的水解性能退化。在这项工作中,通过添加5 wt%膨胀石墨,8 wt% AlCl3和5 wt%聚乙二醇1000,30 wt% Ca-Mg合金氢化板在空气暴露4 h (80 RH%, 25 °C)后,H2产率增加到原始样品的2.3倍而没有任何衰减。此外,当添加15 wt%的聚乙二醇1000时,24 h空气暴露后的衰减率降低了43 %。表征结果表明,Ca4Mg3H14比MgH2更易水解。第一性原理计算表明,由于Ca4Mg3H14对H2O的吸附能更大,Ca4Mg3H14与H2O分子的相互作用更强。通过加入适量的膨胀石墨、AlCl3和聚乙二醇1000,结合压制技术,可以延缓材料与空气的直接接触。同时,膨胀石墨和AlCl3破坏了Mg(OH)2钝化层,提高了材料本身的水解性能,从而共同提高了材料的长期储存性能。本研究对镁钙基水解材料的工程应用具有一定的推动作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
文献相关原料
公司名称
产品信息
阿拉丁
AlCl<sub>3</sub>
阿拉丁
AlCl3
阿拉丁
AlCl3
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


