Bioinformatics and Computationally Supported Redesign of Aspartase for β-Alanine Synthesis by Acrylic Acid Hydroamination
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
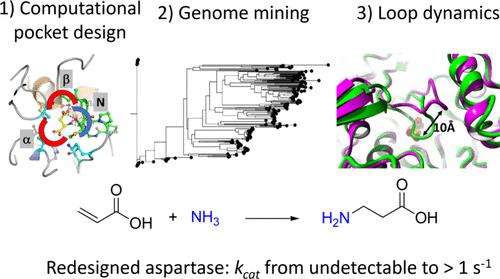
Aspartate ammonia lyases catalyze the reversible amination of fumarate to l-aspartate. Recent studies demonstrate that the thermostable enzyme from Bacillus sp. YM55–1 (AspB) can be engineered for the enantioselective production of substituted β-amino acids. This reaction would be attractive for the conversion of acrylic acid to β-alanine, which is an important building block for the preparation of bioactive compounds. Here we describe a bioinformatics and computational approach aimed at introducing the β-alanine synthesis activity. Three strategies were used: First, we redesigned the α-carboxylate binding pocket of AspB to introduce activity with the acrylic acid. Next, different template enzymes were identified by genome mining, equipped with a redesigned α-carboxylate pocket, and investigated for β-alanine synthesis, which yielded variants with better activity. Third, interactions of the SS-loop that covers the active site and harbors a catalytic serine were computationally redesigned using energy calculations to stabilize reactive conformations and thereby further increase the desired β-alanine synthesis activity. Different improved enzymes were obtained and the best variants showed kcat values with acrylic acid of at least 0.6–1.5 s–1 with KM values in the high mM range. Since the β-alanine production of wild-type enzyme was below the detection limit, this suggests that the kcat/Km was improved by at least 1000-fold. Crystal structures of the 6-fold mutant of redesigned AspB and the similarly engineered aspartase from Caenibacillus caldisaponilyticus revealed that their ligand-free structures have the SS-loop in a closed (reactive) conformation, which for wild-type AspB is only observed in the substrate-bound enzyme. AlphaFold-generated models suggest that other aspartase variants redesigned for acrylic acid hydroamination also prefer a 3D structure with the loop in a closed conformation. The combination of binding pocket redesign, genome mining, and enhanced active-site loop closure thus created effective β-alanine synthesizing variants of aspartase.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


