Elucidating the Reaction Mechanism and Deactivation of CO2-Assisted Propane Oxidative Dehydrogenation over VOx/TiO2 Catalysts: A Multiple Operando Spectroscopic Study
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
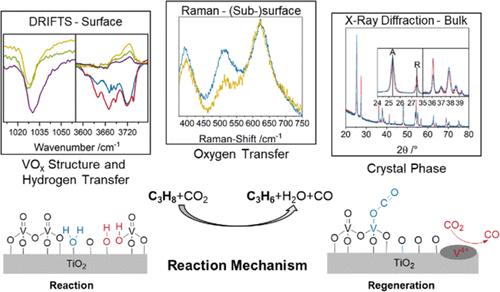
The CO2-assisted oxidative dehydrogenation (ODH) of propane is of great technical importance and enables the use (and thus removal from the atmosphere) of CO2, a greenhouse gas, in a value-adding process. Supported vanadium oxide (VOx) catalysts are a promising alternative to more active but toxic chromium oxide catalysts. Despite its common use, TiO2 has not been investigated as a support material for VOx in the CO2–ODH of propane. In this study, we elucidate the interaction between titania (P25) and vanadia in the reaction mechanism by analyzing the reaction network and investigating the catalyst using X-ray diffraction (XRD), multiwavelength Raman, UV–vis and diffuse reflectance IR Fourier transform (DRIFT) spectroscopy. Besides direct and indirect ODH reaction pathways, propane dry reforming (PDR) is identified as a side reaction, which is more prominent on bare titania. The presence of VOx enhances the stability and the selectivity toward propylene by participating in the redox cycle, activating CO2 and leading to a higher rate of regeneration. Additionally, VOx catalyzes the conversion of anatase to rutile, which facilitates CO2 activation, thereby leading to an encapsulation of vanadium. At higher loadings, reducible VOx oligomers are present on the surface, facilitating some PDR, but less than on bare P25. As the main deactivation mechanisms of the catalyst system, we propose the reduction of the titania lattice and the consumption of vanadium, while carbon formation appears to be less relevant. Our results highlight the importance of analyzing the CO2–ODH reaction network and applying a multispectroscopic approach to obtain a detailed mechanistic understanding of CO2-assisted propane ODH over supported VOx catalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


