Unveiling the Mechanism of Dense Cathode‒Electrolyte Interphase Formation in Lithium-Ion Batteries Using Cyclophosphamide Additive
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
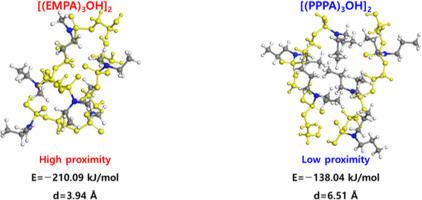
High-voltage lithium-ion batteries (LIBs) have attracted increasing attention for their high energy density. However, at high voltages, cathode degradation and electrolyte decomposition trigger parasitic side reactions that deteriorate battery cycle performance. These issues have been addressed through various studies on cathode‒electrolyte interphase (CEI)-forming additives. In particular, 2-ethylmethylamino-1,3,2-dioxaphospholane 2-oxide (EMPA), a cyclophosphamide (CPA) CEI-forming additive, has shown excellent capacity retention and battery cycle performance at high voltages when added at only 0.5 vol% in LIB systems. However, the molecular-level understanding of CPA additives remains limited. Here, our first-principles calculations reveal that EMPA oxidizes before the solvent in the electrolyte while also scavenging HF and H2O. Specifically, calculations of the dimerization of asymmetric EMPA trimers, represented by two identical [(EMPA)3OH] species forming a [(EMPA)3OH]2 dimer, imply that after oxidation these two identical EMPA polymers bind very strongly and in very close proximity. This was due to the favorable electrostatic interactions with the more widely distributed polar surface in EMPA, in addition to the small number of carbons in the alkyl groups of the amine moiety in CPA. We suggest that the asymmetry in the alkyl groups of the amine moiety in CPA is closely related to the excellent CEI formation observed in the experimental results.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


