Pyroptosis-Inducing Self-Adaptor to Potentiate Immune Checkpoint Blockade Therapy for Breast Cancer by Reeducating the Treatment-Recruited Macrophages
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
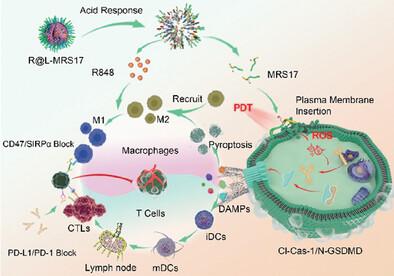
Treatment-induced cell pyroptosis can improve the immunogenicity of breast cancer and enhance the efficacy of immune checkpoint blockade (ICB), but the resultant recruitment of immunosuppressive cells impedes the systemic anti-tumor immunity. Herein, a rationally designed self-adaptor (R@L-MRS17) is developed to initiate breast cancer cell pyroptosis and concomitantly reeducate the pyroptosis-recruited macrophages to enhance the ICB therapy. Of which, R@L-MRS17 promotes breast cancer-specific drug delivery through CD47 recognition, and enables the plasma membrane targeted photosensitizer insertion through hydrophobic and electrostatic interactions. Under light excitation, R@L-MRS17 produces reactive oxygen species (ROS) in situ to trigger cell pyroptosis, followed by the release of pro-inflammatory factors to recruit macrophages and improve tumor immunogenicity. Moreover, the acid responsiveness of R@L-MRS17 facilitates the release of R848 to polarize the infiltrated macrophages into M1 phenotype for active anti-tumor immunity. Additionally, R@L-MRS17 is capable of blocking CD47 to restore the recognition and phagocytosis behavior of M1-type macrophages against breast cancer cells. In short, the stepwise immune activation by R@L-MRS17 significantly suppresses the bilateral tumor growth and metastasis in combination with αPD-L1. This study provides a self-adaptable strategy to activate the immunological cascade, which may spatiotemporally amplify the immune responses for breast cancer.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


