Destabilization of Glycosyl Cation by an Electron-Withdrawing Substituent at C-5 Makes Sialylation Reaction More α-Stereoselective
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Comparison of the reactivity of sialyl chlorides and bromides based on N-acetylneuraminic acid (Neu5Ac) and its deaminated analogue (KDN) in reactions with MeOH and i-PrOH without a promoter revealed that the acetoxy group at C-5 in a molecule of a sialic acid glycosyl donor can destabilize the corresponding glycosyl cation making the SN1-like reaction pathway unfavorable. A change to the SN2-like reaction pathway ensures preferential formation of the α-glycoside.
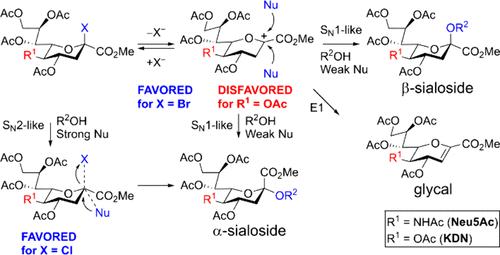
C-5上吸电子取代基对糖基阳离子的破坏使唾液酰化反应更具α-立体选择性
通过比较以 N-乙酰神经氨酸(Neu5Ac)及其脱氨基类似物(KDN)为基础的硅烷基氯化物和溴化物在与不含促进剂的 MeOH 和 i-PrOH 反应中的反应活性,发现硅烷基酸糖基供体分子中 C-5 处的乙酰氧基可破坏相应糖基阳离子的稳定性,从而使 SN1 类反应途径变得不利。改用类似 SN2 的反应途径可确保优先形成 α-糖苷。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


