Discovery of novel capsaicin analogs as TRPV1 inhibitors for the treatment of idiopathic pulmonary fibrosis
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive and fatal interstitial lung disease for which few drugs are available in clinical practice. Here, we identified novel capsaicin analogs by combining in-house chemical library screening and further structural optimization. (E)-1-(3,4-dihydroxyphenyl)-7-phenylhept-1-en-3-one (Compound 14) was found to be the most potent in inhibiting TGF-β-induced collagen accumulation, proliferation and migration in fibroblast cells. Furthermore, compound 14 (IC50 = 0.51 ± 0.06 μM) showed over 100-fold increasing antifibrotic activity compared to capsaicin (IC50 = 53.71 ± 4.78 μM). Notably, compound 14 could target TRPV1, thereby affecting the expression of the fibrosis markers Collagen Ⅰ and α-SMA by inhibiting the TGF-β/Smads and MAPK pathways to exert antifibrotic activity in vitro. Compound 14 significantly inhibited collagen deposition in lung tissues, ameliorated alveolar structures, and increased survival rates in mice with bleomycin-induced pulmonary fibrosis. In addition, compound 14 possessed lower cytotoxicity (compared to nitedanib) and no toxicity in mice. Overall, compound 14 promise as a potential drug candidate for the treatment of IPF.
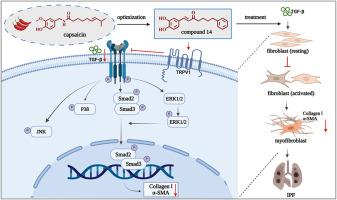

发现新型辣椒素类似物作为TRPV1抑制剂治疗特发性肺纤维化
特发性肺纤维化(IPF)是一种进行性和致死性间质性肺疾病,临床治疗药物很少。在这里,我们通过内部化学文库筛选和进一步的结构优化,确定了新的辣椒素类似物。(E)-1-(3,4-二羟基苯基)-7-苯基庚-1-en-3-one(化合物14)对TGF-β-诱导的成纤维细胞中胶原的积累、增殖和迁移的抑制作用最强。化合物14的抗纤维化活性(IC50 = 0.51±0.06 μM)是辣椒素(IC50 = 53.71±4.78 μM)的100倍以上。值得注意的是,化合物14可以靶向TRPV1,通过抑制TGF-β/Smads和MAPK通路,影响纤维化标志物胶原Ⅰ和α-SMA的表达,从而在体外发挥抗纤维化活性。化合物14显著抑制肺组织胶原沉积,改善肺泡结构,提高博莱霉素诱导肺纤维化小鼠的存活率。此外,化合物14具有较低的细胞毒性(与尼达尼布相比),对小鼠无毒性。总之,化合物14有望成为治疗IPF的潜在候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


