Cooperative Role of Conformation and Ice-Binding Groups in Ice Growth Inhibition of Antifreeze Glycoproteins
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The antifreeze mechanism of antifreeze glycoproteins (AFGPs) remains incompletely understood, which limits the design of new antifreeze molecules for practical applications. For instance, the ice growth inhibition of AFGP8 (the shortest AFGPs) is primarily driven by hydrophobic methyl and hydrogen-bonding hydroxyl groups. However, altering the C3-β linkage in the disaccharide moiety of AFGP8, denoted as variant GP8-LacNAc, significantly reduces its antifreeze activity. This challenges the conventional understanding of the antifreeze activity of AFGP8 because no group is removed. Here, we revisit the antifreeze mechanism using molecular dynamics simulations of two AFGPs as an example, revealing the relation between conformation, ice-binding group, and ice growth inhibition activity. The PPII helix is not the reason for the weak activity of GP8-LacNAc, while the cooperativity of the conformation and group matters. AFGP8 maintains a dominant conformation with all four disaccharides on the same side, which maximizes the binding with ice. While GP8-LacNAc undergoes frequent conformational transitions, leading to a methyl-hydroxyl distance mismatch with the ice surface. Our results reveal the cooperative role of conformation and ice-binding groups, providing new insights into the antifreeze mechanism of flexible proteins.
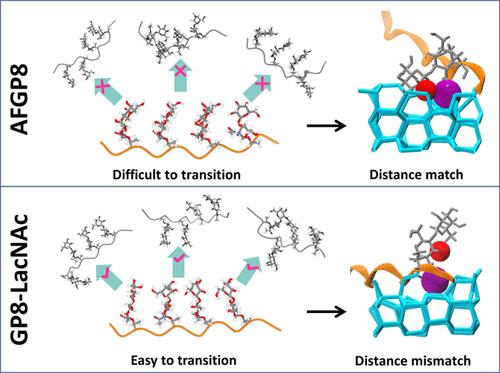
抗冻糖蛋白的构象和冰结合基团在冰生长抑制中的协同作用
抗冻糖蛋白(AFGPs)的抗冻机制尚不完全清楚,这限制了新型抗冻分子的实际应用设计。例如,AFGP8(最短的AFGPs)的冰生长抑制主要是由疏水甲基和氢键羟基驱动的。然而,改变AFGP8双糖部分的C3-β链(称为变体GP8-LacNAc)会显著降低其抗冻活性。这挑战了对AFGP8抗冻活性的传统理解,因为没有移除任何基团。本文以两种afgp的分子动力学模拟为例,回顾了抗冻机制,揭示了构象、冰结合基团和冰生长抑制活性之间的关系。PPII螺旋不是GP8-LacNAc活性弱的原因,而构象和基团的协同作用是重要的。AFGP8与所有四种双糖在同一侧保持优势构象,从而最大限度地与冰结合。而GP8-LacNAc经历频繁的构象转变,导致甲基-羟基与冰表面的距离不匹配。我们的研究结果揭示了构象和冰结合基团的协同作用,为柔性蛋白的抗冻机制提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


