Nickel-Based Hollow Spheres with Optimized Interfacial Electronic Structures by Highly Dispersed MoN for Efficient Urea Electrolysis
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
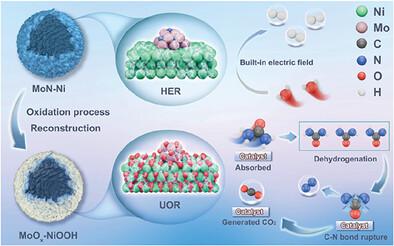
Ni-Mo-based catalysts that exhibit well-synergized and readily accessible catalytic sites are ideal catalysts for achieving efficient electrocatalysis. Herein, the synthesis of hollow Ni spheres with a hierarchical nanosheet surface modified by highly dispersed MoN for efficient urea electrolysis is reported. This synthesis is based on the design of hollow Mo-Ni precursors featuring a nanosheet array surface, achieved through the phosphomolybdic acid (PMo12)-mediated reconstruction of hollow Ni-BTC spheres. The optimized MoN-Ni catalyst can effectively drive both the urea oxidation reaction (UOR) and hydrogen evolution reaction at low potentials of 1.37 V and 191 mV, respectively, achieving a current density of 100 mA cm−2. The electrolytic cell utilizing these catalysts can sustain 100 mA cm−2 at a low voltage of 1.53 V and can operate continuously for over 220 h. The X-ray photoelectron spectroscopy (XPS) and density functional theory (DFT) analyses demonstrate the established built-in electric field facilitates electron transfer from MoN to Ni, optimizing the d-band center and consequently reducing the reaction barrier for the UOR. In situ electrochemical impedance spectroscopy (EIS) and in situ Fourier-transform infrared spectroscopy analyses indicate that MoN promotes the formation of high-valent Ni sites, which accelerates the UOR and facilitates the urea eletrolysis through a more environmentally friendly “carbonate” pathway.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


