Role of centchroman in regression of fibroadenoma: A 2-arm randomized control trial
IF 2.4
4区 医学
Q2 MEDICINE, GENERAL & INTERNAL
引用次数: 0
Abstract
Background
Fibroadenomas are common benign breast lumps that can cause anxiety due to malignancy concerns, and Centchroman, a selective estrogen receptor modulator, has shown promise in reducing their size. This study aimed to evaluate the efficacy of Centchroman in reducing fibroadenoma size, mastalgia, anxiety, and depression in affected patients.
Methodology
A parallel-arm randomized controlled trial was conducted at a tertiary care Breast Clinic with 104 patients aged 18‒45 years having fibroadenomas ≤ 3 cm. Group A received Centchroman (30 mg on alternate days) and Group B received a placebo for 12 weeks. The primary outcome was fibroadenoma volume reduction measured by ultrasound, while secondary outcomes included reductions in mastalgia and improvements in anxiety and depression (HADS scores).
Results
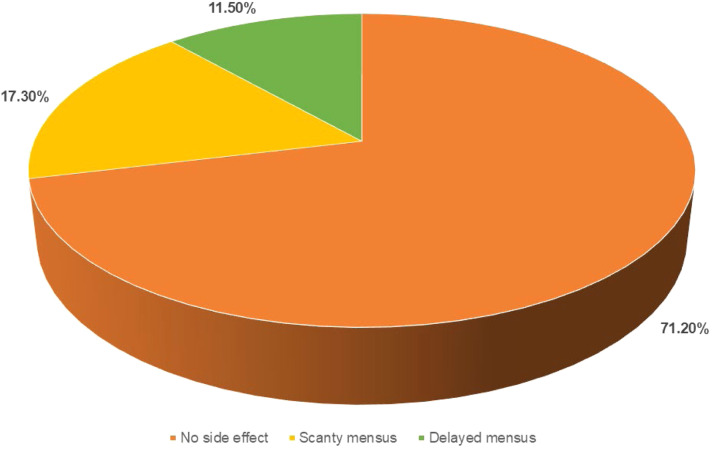
Both groups showed fibroadenoma volume reduction over 12-weeks, with a greater reduction in the intervention group (3.67 ± 1.65 cm3 to 2.29 ± 1.04 cm3) than the control group (3.12 ± 1.16 cm3 to 2.73 ± 0.78 cm3), though the difference was not statistically significant (p = 0.342 and p = 0.781). However, 28.8 % of the intervention group experienced over 50 % volume reduction compared to 13.5 % in the control group (p = 0.007). Mastalgia significantly improved in the intervention group (VAS: 5.76 ± 2.13 to 2.24 ± 0.93, p = 0.023), with minimal improvement in controls. Anxiety and depression scores significantly decreased in the intervention group, with anxiety dropping from 9 to 5 and depression from 6 to 4 (p = 0.001).
Conclusion
Centchroman effectively reduces fibroadenoma size especially those with multiple fibroadenoma, mastalgia, and psychological distress in patients with fibroadenoma.
centchroman在纤维腺瘤消退中的作用:一项两组随机对照试验。
背景:纤维腺瘤是一种常见的良性乳腺肿块,可引起因恶性肿瘤引起的焦虑,而选择性雌激素受体调节剂Centchroman已显示出缩小其大小的希望。本研究旨在评估Centchroman在减少纤维腺瘤大小、乳房痛、焦虑和抑郁方面的疗效。方法:在一家三级乳腺诊所进行了一项平行组随机对照试验,纳入了104例年龄在18-45岁、纤维腺瘤≤3 cm的患者。A组服用Centchroman (30 mg,隔天服用),B组服用安慰剂,疗程12周。主要结果是超声测量纤维腺瘤体积缩小,次要结果包括乳房痛的减少和焦虑和抑郁的改善(HADS评分)。结果:两组患者12周内纤维腺瘤体积均缩小,干预组缩小幅度(3.67±1.65 cm3至2.29±1.04 cm3)大于对照组(3.12±1.16 cm3至2.73±0.78 cm3),但差异无统计学意义(p = 0.342和p = 0.781)。然而,28.8%的干预组经历了超过50%的体积缩小,而对照组为13.5% (p = 0.007)。干预组乳痛明显改善(VAS: 5.76±2.13 ~ 2.24±0.93,p = 0.023),对照组改善最小。干预组焦虑和抑郁得分显著下降,焦虑从9分降至5分,抑郁从6分降至4分(p = 0.001)。结论:Centchroman能有效地减少纤维腺瘤的大小,特别是对多发纤维腺瘤、乳房疼痛和纤维腺瘤患者的心理困扰。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Clinics
医学-医学:内科
CiteScore
4.10
自引率
3.70%
发文量
129
审稿时长
52 days
期刊介绍:
CLINICS is an electronic journal that publishes peer-reviewed articles in continuous flow, of interest to clinicians and researchers in the medical sciences. CLINICS complies with the policies of funding agencies which request or require deposition of the published articles that they fund into publicly available databases. CLINICS supports the position of the International Committee of Medical Journal Editors (ICMJE) on trial registration.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


