Novel antimalarial 3-substituted quinolones isosteres with improved pharmacokinetic properties
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Aryl quinolone derivatives can target the cytochrome bc1 complex of Plasmodium falciparum, exhibiting excellent in vitro and in vivo antimalarial activity. However, their clinical development has been hindered due to their poor aqueous solubility profiles. In this study, a series of bioisosteres containing saturated heterocycles fused to a 4-pyridone ring were designed to replace the inherently poorly soluble quinolone core in antimalarial quinolones with the aim to reduce π-π stacking interactions in the crystal packing solid state, and a synthetic route was developed to prepare these alternative core derivatives. One such novel derivate, F14, exhibited significant enhancements in both aqueous solubility (20 μM) and lipophilicity (LogD 2.7), whilst retaining nanomolar antimalarial activity against the W2 strain of P. falciparum (IC50 = 235 nM). The pharmacokinetic studies reported, provide preliminary insights into the in vivo distribution and elimination of F14, while findings from single crystal X-ray diffraction experiment rationalized the enhanced solubility. Protein X-ray crystallography and in silico docking simulations provide insight into the potential mode of action within the cytochrome bc1 complex. These findings demonstrated the viability of this bioisostere replacement strategy and provided support for further exploration of in vivo efficacy in preclinical animal models and valuable insights for new drug design strategies in the fight against malaria.
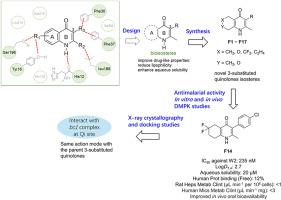

具有改进药代动力学性质的新型抗疟3-取代喹诺酮类异甾体
芳基喹诺酮类衍生物可靶向恶性疟原虫细胞色素bc1复合体,具有良好的体内体外抗疟活性。然而,由于其水溶性较差,其临床发展受到阻碍。本研究设计了一系列含饱和杂环与4-吡啶酮环融合的生物异构体,以取代抗疟喹诺酮类药物中固有的难溶性喹诺酮核心,以减少晶体包装固体中π-π堆叠相互作用,并开发了一种合成路线来制备这些替代核心衍生物。其中一种新型衍生物F14在水溶性(20 μM)和亲脂性(LogD 2.7)方面均表现出显著增强,同时对恶性疟原虫W2菌株(IC50 = 235 nM)保持纳摩尔抗疟活性。所报道的药代动力学研究为F14在体内的分布和消除提供了初步的见解,而单晶x射线衍射实验的发现合理化了F14增强的溶解度。蛋白质x射线晶体学和硅对接模拟提供了对细胞色素bc1复合物内潜在作用模式的深入了解。这些发现证明了这种生物同位体替代策略的可行性,为进一步探索临床前动物模型的体内疗效提供了支持,并为抗疟疾新药设计策略提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


