Synthesis and functional screening of novel inhibitors targeting the HDAC6 zinc finger ubiquitin-binding domain
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
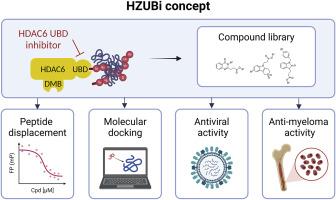
Histone deacetylase 6 (HDAC6) is a promising target for treating neurodegenerative disorders, several cancer types and viral infections. Unique among HDACs, the HDAC6 isoform possesses a zinc finger ubiquitin-binding domain (UBD) crucial for managing misfolded protein aggregates and facilitating viral infection. HDAC6 binds aggregated polyubiquitinated proteins through its UBD, mediating their transport to the aggresome and subsequent removal via autophagy. Despite the importance of the UBD in proteostasis and viral infection, its pharmacological inhibition has been minimally explored thus far, with research largely focused on the deacetylase domain. We synthesized a diverse library of new compounds designed to target the HDAC6-UBD, termed HZUBi, with varied core structures including quinazolinone, oxindole and tetrahydrothiopyrano[4,3-b]indole, aimed at enhancing UBD interaction and extending into the side pocket. New structure-activity relationships were established, computational docking and molecular dynamics studies were performed and the functional impact of selected inhibitors was assessed in the context of multiple myeloma and viral infection. Several new HZUBi could displace a ubiquitin peptide from HDAC6-UBD in a differential manner, although to a lower extent than the literature reference compound HZUBi-3e. Despite exhibiting in vitro target engagement, neither HZUBi-3e nor its ester prodrug HZUBi-1e enhanced proteasome inhibitor-mediated multiple myeloma cell killing. Finally, none of the screened HZUBi triggered anti-viral activity.

新型HDAC6锌指泛素结合域抑制剂的合成及功能筛选
组蛋白去乙酰化酶6 (HDAC6)是治疗神经退行性疾病、几种癌症类型和病毒感染的有希望的靶点。在hdac中,独特的HDAC6异构体具有锌指泛素结合结构域(UBD),对管理错误折叠的蛋白质聚集和促进病毒感染至关重要。HDAC6通过其UBD结合聚集的多泛素化蛋白,介导它们转运到聚合体并随后通过自噬去除。尽管UBD在蛋白酶抑制和病毒感染中具有重要作用,但迄今为止对其药理抑制作用的探索很少,研究主要集中在去乙酰化酶结构域。我们合成了多种针对HDAC6-UBD的新化合物,称为HZUBi,其核心结构包括喹唑啉酮、氧吲哚和四氢硫代吡喃[4,3-b]吲哚,旨在增强UBD相互作用并延伸到侧袋。建立了新的构效关系,进行了计算对接和分子动力学研究,并在多发性骨髓瘤和病毒感染的背景下评估了选定抑制剂的功能影响。几种新的HZUBi可以以不同的方式取代HDAC6-UBD中的一个泛素肽,尽管程度低于文献参考化合物HZUBi-3e。尽管HZUBi-3e及其酯前药HZUBi-1e表现出体外靶标结合,但都没有增强蛋白酶体抑制剂介导的多发性骨髓瘤细胞杀伤。最后,筛选的HZUBi都没有触发抗病毒活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


