Synthesis of Atropisomeric Quinazolin-4-one Derivatives Based on Remote H/D and 12C/13C Discrimination
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
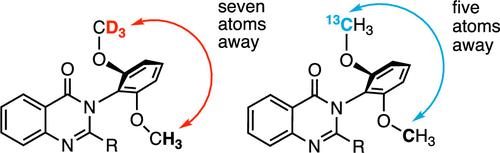
Both enantiomers of 2-ethylquinazolin-4-ones bearing ortho-CH3O/CD3O and CH3O/13CH3O phenyl groups at the N3 position were prepared. These are isotopic atropisomeric compounds based on a remote and conformationally flexible H/D and 12C/13C discrimination, and it was found that a CHCl3 solution of ortho-CH3O/CD3O derivative shows a slight specific optical rotation. Furthermore, diastereomeric quinazolinone derivatives bearing a chiral carbon were prepared, and their stereochemical purities and rotational stability as well as the isotopic atropisomerism were verified by 1H NMR and chiral high-performance liquid chromatography (HPLC) analyses.
基于远程H/D和12C/13C判别的Atropisomeric Quinazolin-4-one衍生物的合成
2-乙基喹唑啉-4-酮的对映体在N3位置上分别含有邻ch30 / cd30和ch30 / 13ch30苯基。这些是基于远程和构象灵活的H/D和12C/13C辨别的同位素异位异构体化合物,并且发现邻位ch30 / cd30衍生物的CHCl3溶液具有轻微的比旋光性。此外,制备了含有手性碳的非对映体喹唑啉酮衍生物,并通过1H NMR和手性高效液相色谱(HPLC)分析验证了其立体化学纯度、旋转稳定性和同位素反异构性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


