Comparative Study on the Effect of Ethylene Cofeeding in CO2 and CO Hydrogenation to Olefins over FeZnNa Catalyst
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
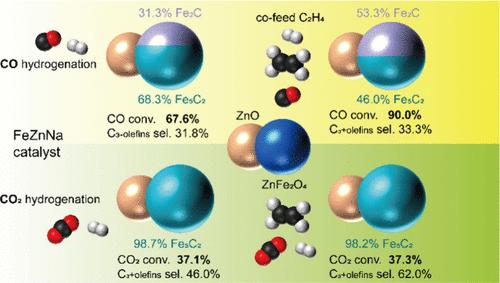
The hydrogenation of CO and CO2 to long-chain olefins presents a promising route for chemical production, but optimizing the reaction process requires a thorough understanding of the tail gas recycling process. The effects of cofeeding ethylene on the hydrogenation of CO and CO2 using a zinc- and sodium-promoted iron catalyst (FeZnNa catalyst) are carefully investigated in this work. For CO2 hydrogenation, ethylene showed negligible impact on CO2 conversion, CO selectivity, or CH4 selectivity but primarily served as a feedstock for the production of ethane and higher carbon number olefins. In contrast, during CO hydrogenation, CO conversion improved with ethylene cofeeding. Ethylene also contributed to chain growth, although a higher fraction was converted to ethane via hydrogenation compared to CO2 hydrogenation. Structural analysis using XRD and Mössbauer spectroscopy revealed that the catalyst in CO2 hydrogenation consisted exclusively of the Fe5C2 phase, whereas CO hydrogenation resulted in the formation of both Fe5C2 and Fe2C phases. XPS and TPO analyses indicated significantly lower carbon deposition on the catalyst during CO2 hydrogenation compared to that during CO hydrogenation.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


