Borohydride oxidation over Pt/C, Au/C and AuPt/C thin-film electrodes studied by rotating disk electrode and differential electrochemical mass spectrometry flow cell measurements
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
We report results of a systematic study of the borohydride oxidation reaction (BOR) in borohydride containing 0.5 M NaOH electrolyte over Pt/C, Au/C and AuPt/C catalyst thin-film electrodes, performed under enforced mass transport conditions. Employing rotating disk electrode (RDE) and thin-layer flow cell differential electrochemical mass spectrometry (DEMS) measurements, we identify kinetic limitations over a wide range of transport conditions. Together with the highly sensitive detection of evolved hydrogen as a function of potential, due to the use of a cold trap at the mass spectrometer inlet, this allows us to separate changes in the reaction selectivity, from complete to incomplete borohydride oxidation, from other kinetic limitations. Evaluation of the (apparent) number of electrons transferred per borohydride ion, both from the RDE measurements via the Koutecky-Levich formalism and from the DEMS measurements via the H2 formation current, further supports the identification of complete borohydride oxidation (8 electron transfer) and incomplete oxidation (< 8 electrons transfer) reaction conditions. Using data on isotope labeled BD4- oxidation that we had published earlier, we identify weak secondary kinetic isotope effects for all catalysts, which indicate that B-H bond breaking does not represent the rate limiting step.
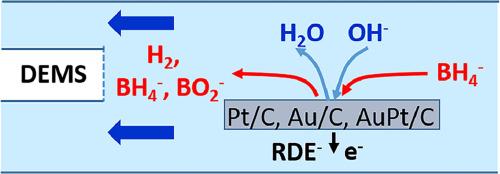
通过旋转圆盘电极和微分电化学质谱流动电池测量,研究了铂/C、Au/C和AuPt/C薄膜电极上硼氢化物的氧化
我们报告了在强制质量输运条件下,在含有0.5 M NaOH电解质的硼氢化物中,在Pt/C、Au/C和AuPt/C催化剂薄膜电极上进行硼氢化物氧化反应(BOR)的系统研究结果。采用旋转圆盘电极(RDE)和薄层流动电池差分电化学质谱(dem)测量,我们确定了在广泛的运输条件下的动力学限制。由于在质谱仪入口使用了冷阱,因此可以高度灵敏地检测出作为电位函数的演化氢,这使我们能够将反应选择性的变化,从完全到不完全硼氢化物氧化,从其他动力学限制中分离出来。通过Koutecky-Levich形式的RDE测量和通过H2形成电流的dem测量,对每个硼氢化物离子转移的(表观)电子数进行了评估,进一步支持了硼氢化物完全氧化(8个电子转移)和不完全氧化(<;8 .电子转移)反应条件。利用我们之前发表的同位素标记BD4-氧化的数据,我们确定了所有催化剂的弱次级动力学同位素效应,这表明B-H键断裂并不代表限速步骤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


