A fluorinated hydrophobic metal–organic framework for CH4 purification from seven-component C1/C2/C3 hydrocarbons mixture
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
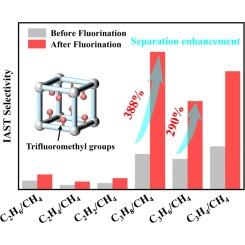
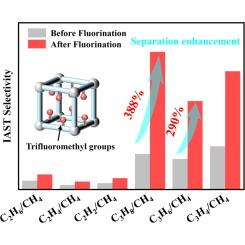
The efficient separation of light hydrocarbons from natural gas is crucial for natural gas purification, yet it remains a challenging and energy-intensive process. Adsorbent-based gas separation methods using metal–organic frameworks (MOFs) offer potential advantages, such as simplicity and energy efficiency. In this study, a pillar-layered MOF, Ni-FDMOF, was constructed by introducing trifluoromethyl (−CF3) groups into the Ni-DMOF framework to modify the pore environment. The C2 and C3 recognition ability and hydrophobicity of Ni-FDMOF were investigated. Single-component adsorption tests and ideal adsorption solution theory (IAST) calculations confirmed that Ni-FDMOF exhibited higher selectivity for C2/CH4 and C3/CH4 separation than Ni-DMOF. Notably, Ni-FDMOF achieved an impressive C3/CH4 selectivity of approximately 100, ranking among the top recorded MOFs. Theoretical calculations indicated that multiple attractive interactions between C2-C3 molecules and −CF3 groups play a crucial role in enhancing the adsorption of C2-C3. Moisture stability experiments demonstrated that Ni-FDMOF is a robust MOF with low water vapor capacity, due to the presence of hydrophobic −CF3 groups. Transient breakthrough simulations further verified the excellent separation of C1/C2/C3 seven-component mixtures by Ni-FDMOF, highlighting its potential for industrial applications.
用于从七组分C1/C2/C3碳氢化合物混合物中纯化CH4的氟化疏水金属-有机框架
从天然气中有效分离轻烃对天然气净化至关重要,但这仍然是一个具有挑战性和能源密集型的过程。基于吸附剂的气体分离方法使用金属有机框架(MOFs)具有潜在的优势,如简单和节能。在本研究中,通过在Ni-DMOF框架中引入三氟甲基(−CF3)基团来修饰孔环境,构建了柱状层状的MOF Ni-FDMOF。研究了Ni-FDMOF对C2和C3的识别能力和疏水性。单组分吸附试验和理想吸附溶液理论(IAST)计算证实,Ni-FDMOF对C2/CH4和C3/CH4的选择性高于Ni-DMOF。值得注意的是,Ni-FDMOF实现了令人印象深刻的C3/CH4选择性,约为100,在记录的mof中名列前茅。理论计算表明,C2-C3分子与−CF3基团之间的多重吸引相互作用对增强C2-C3的吸附起着至关重要的作用。水分稳定性实验表明,Ni-FDMOF是一种稳定的MOF,由于疏水- CF3基团的存在,具有较低的水蒸气容量。瞬态突破模拟进一步验证了Ni-FDMOF对C1/C2/C3七组分混合物的分离效果,凸显了其工业应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


