Personalized Vascularized Tumor Organoid‐on‐a‐Chip for Tumor Metastasis and Therapeutic Targeting Assessment
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
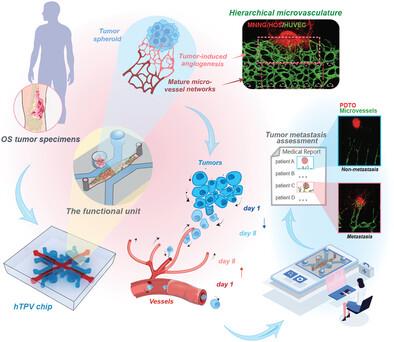
While tumor organoids have revolutionized cancer research by recapitulating the cellular architecture and behaviors of real tumors in vitro, their lack of functional vasculature hinders their attainment of full physiological capabilities. Current efforts to vascularize organoids are struggling to achieve well‐defined vascular networks, mimicking the intricate hierarchy observed in vivo, which restricts the physiological relevance particularly for studying tumor progression and response to therapies targeting the tumor vasculature. An innovative vascularized patient‐derived tumor organoids (PDTOs)‐on‐a‐chip with hierarchical, tumor‐specific microvasculature is presented, providing a versatile platform to explore tumor‐vascular dynamics and antivascular drug efficacy. It is found that highly metastatic tumor cells induced vessel angiogenesis and simultaneously migrated toward blood vessels via the Notch pathway. The evident association between the angiogenic and migratory capacities of PDTOs and their clinical metastatic outcomes underscores the potential of the innovative platform for evaluating tumor metastasis, thus offering valuable insights for clinical decision‐making. Ultimately, the system represents a promising avenue for advancing the understanding of tumor metastasis and developing personalized treatment strategies based on patient‐specific tumor characteristics.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


