Splenic fibroblasts control marginal zone B cell movement and function via two distinct Notch2-dependent regulatory programs
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Innate-like splenic marginal zone (MZ) B (MZB) cells play unique roles in immunity due to their rapid responsiveness to blood-borne microbes. How MZB cells integrate cell-extrinsic and -intrinsic processes to achieve accelerated responsiveness is unclear. We found that Delta-like1 (Dll1) Notch ligands in splenic fibroblasts regulated MZB cell pool size, migration, and function. Dll1 could not be replaced by the alternative Notch ligand Dll4. Dll1-Notch2 signaling regulated a Myc-dependent gene expression program fostering cell growth and a Myc-independent program controlling cell-movement regulators such as sphingosine-1 phosphate receptor 1 (S1PR1). S1pr1-deficient B cells experienced Notch signaling within B cell follicles without entering the MZ and were retained in the spleen upon Notch deprivation. Key elements of the mouse B cell Notch regulome were preserved in subsets of human memory B cells and B cell lymphomas. Thus, specialized niches program the poised state and patrolling behavior of MZB cells via conserved Myc-dependent and Myc-independent Notch2-regulated mechanisms.
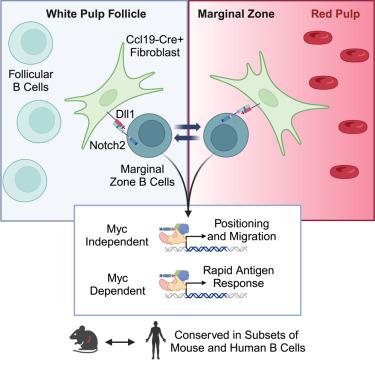
脾成纤维细胞通过两个不同的notch2依赖性调节程序控制边缘区B细胞的运动和功能
先天样脾边缘带B (MZB)细胞对血源性微生物的快速反应在免疫中发挥着独特的作用。MZB细胞如何整合细胞外源性和内源性过程以实现加速反应尚不清楚。我们发现脾脏成纤维细胞中的Delta-like1 (Dll1) Notch配体调节MZB细胞池的大小、迁移和功能。Dll1不能被Notch配体Dll4替代。Dll1-Notch2信号调节myc依赖性基因表达程序促进细胞生长和myc独立程序控制细胞运动调节剂,如鞘氨醇-1磷酸受体1 (S1PR1)。s1pr1缺陷的B细胞在B细胞滤泡内经历Notch信号传导而不进入MZ,并且在Notch缺失后保留在脾脏中。小鼠B细胞Notch规则组的关键元件在人类记忆B细胞和B细胞淋巴瘤亚群中被保留。因此,通过保守的myc依赖性和myc非依赖性notch2调节机制,专门的生态位调控了MZB细胞的平衡状态和巡逻行为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


