Transposon-triggered epigenetic chromatin dynamics modulate EFR-related pathogen response
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
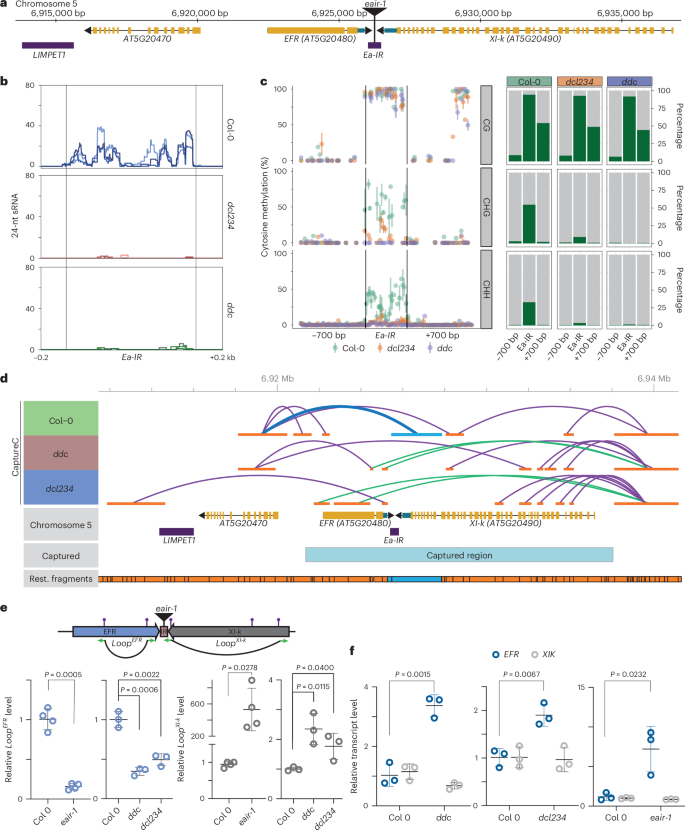
Infectious diseases drive wild plant evolution and impact crop yield. Plants, like animals, sense biotic threats through pattern recognition receptors (PRRs). Overly robust immune responses can harm plants; thus, understanding the tuning of defense response mechanisms is crucial for developing pathogen-resistant crops. In this study, we found that an inverted-repeat transposon (EFR-associated IR, Ea-IR) located between the loci encoding PRRs ELONGATION FACTOR-TU RECEPTOR (EFR) and myosin XI-k (XI-k) in Arabidopsis affects chromatin organization, promoting the formation of a repressive chromatin loop. Upon pathogen infection, chromatin changes around EFR and XI-k correlate with increased EFR transcription. Pathogen-induced chromatin opening causes RNA polymerase II readthrough, producing a longer, Ea-IR-containing XI-k transcript, processed by Dicer-like enzymes into small RNAs, which reset chromatin to a repressive state attenuating the immune response after infection. Arabidopsis accessions lacking Ea-IR have higher basal EFR levels and resistance to pathogens. We show a scenario in which a transposon, chromatin organization and gene expression interact to fine-tune immune responses, during both the course of infection and the course of evolution. Here, the authors show that an inverted-repeat transposon located next to the pattern recognition receptor ELONGATION FACTOR-TU RECEPTOR (EFR)-encoding gene in Arabidopsis controls chromatin organization, EFR gene expression and plant immune response.

转座子触发的表观遗传染色质动力学调节efr相关病原体反应
传染病推动野生植物进化,影响作物产量。植物和动物一样,通过模式识别受体(PRRs)感知生物威胁。过度强烈的免疫反应会伤害植物;因此,了解防御反应机制的调整对开发抗病作物至关重要。在本研究中,我们发现拟南芥中位于编码PRRs的伸长因子- tu受体(EFR)和肌球蛋白XI-k (XI-k)位点之间的倒置重复转座子(EFR-associated IR, Ea-IR)影响染色质组织,促进抑制染色质环的形成。在病原体感染后,EFR和XI-k周围的染色质变化与EFR转录增加相关。病原体诱导的染色质打开导致RNA聚合酶II读取,产生更长的含有ea - ir的XI-k转录物,由dicer样酶加工成小RNA,将染色质复位到抑制状态,从而减弱感染后的免疫反应。缺乏Ea-IR的拟南芥材料具有较高的基础EFR水平和对病原体的抗性。我们展示了转座子、染色质组织和基因表达在感染和进化过程中相互作用以微调免疫反应的场景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


