Commensal-pathogen dynamics structure disease outcomes during Clostridioides difficile colonization
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Gastrointestinal colonization by Clostridioides difficile is common in healthcare settings and ranges in presentation from asymptomatic carriage to lethal C. difficile infection (CDI). We used a systems biology approach to investigate why patients colonized with C. difficile have a range of clinical outcomes. Microbiota humanization of germ-free mice with fecal samples from toxigenic C. difficile carriers revealed a spectrum of virulence among clinically prevalent clade 1 lineages and identified candidate taxa, including Blautia, as markers of stable colonization. Using gnotobiotic mice engrafted with defined human microbiota, we validated strain-specific CDI severity across clade 1 strains isolated from patients. Mice engrafted with a community broadly representative of colonized patients were protected from severe disease across all strains without suppression of C. difficile colonization. These results underline the capacity of gut community structure to attenuate a diversity of pathogenic strains without inhibiting colonization, providing insight into determinants of stable C. difficile carriage.
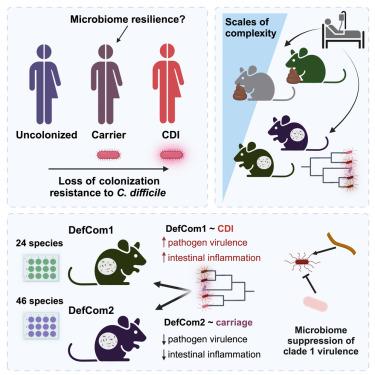
在艰难梭菌定植期间,共生病原体动力学结构疾病结果
艰难梭菌的胃肠道定植在医疗机构中很常见,其表现形式从无症状携带到致命的艰难梭菌感染(CDI)。我们使用系统生物学方法来研究为什么患者定殖艰难梭菌有一系列的临床结果。用产毒艰难梭菌携带者的粪便样本将无菌小鼠的微生物群人源化,揭示了临床流行的进化枝1谱系的毒力谱,并确定了候选分类群,包括蓝芽胞杆菌,作为稳定定植的标志。利用植入了特定人类微生物群的小鼠,我们验证了从患者分离的进化支1菌株的菌株特异性CDI严重程度。移植具有广泛代表性的定植患者群体的小鼠在没有抑制艰难梭菌定植的情况下免受所有菌株的严重疾病。这些结果强调了肠道群落结构在不抑制定植的情况下减弱致病性菌株多样性的能力,从而深入了解了艰难梭菌稳定携带的决定因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


