Visible-Light Induced and Iron Peroxo-Promoted Radical Difunctionalization of Alkene for the Synthesis of β-Ketosulfone and α-Chloroketone
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
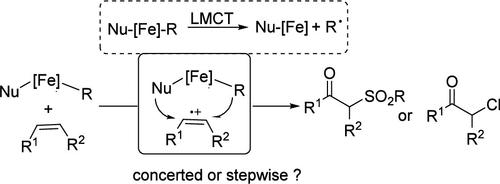
In this work, a switchable synthesis of β-ketosulfone and α-chloroketone through a radical difunctionalization of alkenes is reported. The transformation works well under iron peroxo species/photoredox dual catalysis and an open-flask atmosphere, and the reaction is highlighted with good yields and a broad reaction scope. Mechanism studies show that the reaction is initiated by a formal [4 + 2] cyclization of the sulfonyl radical in a regioselective manner.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


