Emergence of Tetragonal Phase and Reentrant Transition in Tensile-Strained Bi2(La1–xBix)O4Cl Solid Solution
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
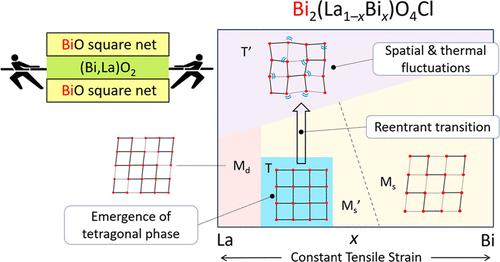
Manipulating chemical bonding in a solid is crucial for controlling and realizing desirable properties. We have recently demonstrated that replacing the M3+ cation of Bi2MO4Cl, which contains triple-fluorite slabs, from Y3+ to a larger cation (La3+, Bi3+) induces tensile strain in the Bi–O square net, resulting in Bi–O bond cleavage to form double- and single-chain structures, respectively. In this study, we synthesized a solid solution of Bi2(La1–xBix)O4Cl with almost uniform tensile strain, revealing an unexpected tetragonal (T) phase (0.15 ≤ x ≤ 0.35), along with an additional monoclinic single-chain phase (0.425 ≤ x ≤ 0.475). The emergence of the T phase with the elongated in-plane axis likely arises from the competition between the single and double chain structures. The T phase exhibits a narrower bandgap of 2.2 eV (vs Bi2YO4Cl), ascribed to the presence of Bi in the inner sublayer. Furthermore, the T phase undergoes a reentrant transition upon heating via the single-chain phase, forming a high-temperature tetragonal (T′) phase with partial Bi–O bond cleavage due to spatial and thermal fluctuations of the outer Bi cations. This study emphasizes the role of uniform tensile strain in promoting phase competition in triple fluorite layered systems, resulting in complex phase transitions under external stimuli. Controlling such delicate balance offers a pathway to engineering of physical properties of functional materials, such as visible-light photocatalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


