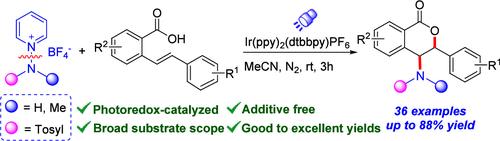
Photoredox-Catalyzed Aminolactonization of 2-Styrylbenzoic Acids with N-Aminopyridinium Salts to Access 4-Sulfonamino-3,4-dihydroisocoumarins
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A photoredox-catalyzed aminolactonization of unsaturated carboxylic acids was achieved using N-aminopyridinium salts as the amino radical precursor. The transformation features mild conditions and a remarkably broad substrate scope, offering an efficient approach to construct a wide range of 4-sulfonamino 3,4-dihydroisocoumarins. Mechanistic studies indicate that the reaction proceeds via a distinctive N-aminopyridinium salt-promoted electrophilic amination of 2-styrylbenzoic acids.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


