Unique Cux+/Cu0 active-site switches in Cu-loaded g-C3N4 nanosheets for efficient photocatalytic CO2 reduction
IF 11.2
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
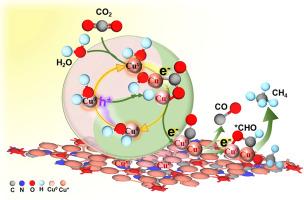
Cu metal and its oxides have attracted much attention for photocatalytic CO2 reduction reaction (CO2RR), but the stability and effects of Cu oxidation states on CO2RR are not fully understood. Cux+/Cu0-loaded graphitic carbon nitride (g-C3N4) heterojunctions (Cu-CuOx/g-C3N4) are fabricated via a stepwise calcination method for efficient photocatalytic CO2RR. Cu2O is the main component of Cu-CuOx and the mixed valence Cu includes Cu0, Cu+, and Cu2+, which play the role of charge trapping sites and redox catalytic centers during the photocatalytic CO2RR process. The main products were CO and CH4 for the CO2RR with production rates of 14.45 and 0.66 μmol g−1 h−1 for CO and CH4, which were higher than those for g-C3N4 and Cu-CuOx, respectively. This photocatalytic CO2RR performance is attributed to the ultrafast switching of “Cux+−Cu0” and eCB−/hVB+ trapping transformation in Cu-CuOx benefited from the built-in IEF between Cu-CuOx and g-C3N4, increasing the efficient photogenerated eCB−, and enabling the stability of Cu-CuOx/g-C3N4. Cux+ adsorbed by H2O works as the electron trapping site to change to Cu0 and switch to the hole trapping site; Cu0 works as the hole trapping site to change to Cux+ and switch to the electron trapping site, causing the CO2RR of the adsorbed CO2. Moreover, the coordinated Cu0 and Cu+ species facilitate the activation of the adsorbed CO2 and *CO generation, these adsorbed *CO on Cu0 and Cu+ detected by in-situ DRIFTS quickly transformed to *CHO with a lower energy barrier benefited from the mixed Cu0/Cu+ active sites during CORR to produce CH4. This finding provides a new insight into the influence of mixed valence Cu during photocatalytic CO2RR.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Science & Technology
工程技术-材料科学:综合
CiteScore
20.00
自引率
11.00%
发文量
995
审稿时长
13 days
期刊介绍:
Journal of Materials Science & Technology strives to promote global collaboration in the field of materials science and technology. It primarily publishes original research papers, invited review articles, letters, research notes, and summaries of scientific achievements. The journal covers a wide range of materials science and technology topics, including metallic materials, inorganic nonmetallic materials, and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


