Cobalt(II) Aqua Complex-Mediated Hydrogen Peroxide Activation: Possible Roles of HOOOH and Co(II)–OOOH Intermediates in Singlet Oxygen Generation
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
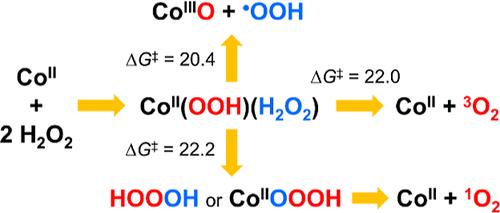
Density functional theory (DFT) calculations indicate that [CoII(H2O)6]2+ reacts with two H2O2 molecules to form [(H2O)4CoII(OOH)(H2O2)]+ reactant complexes, which decompose through three distinct pathways depending on the relative orientation between the coordinated –OOH and H2O2 ligands. The reactive intermediates produced via these activation pathways include hydroperoxyl (•OOH)/superoxide (O2•–) radicals, singlet oxygen (1O2), and Co(III) species [(H2O)5CoIII(O)]+, [(H2O)4CoIII(OH)2]+, and [(H2O)5CoIII(OH)]2+. The Co(III) species display from moderate to strong oxidizing abilities that have long been overlooked. Remarkably, our DFT calculations reveal the possible formation of hydrogen trioxide (HOOOH) and Co(II)–OOOH intermediates during [(H2O)4CoII(OOH)(H2O2)]+ decomposition and that the hydrolysis of these transient species is a route to 1O2 production. Because two of the three activation pathways do not involve changes in the oxidation state of the Co center, they may apply to other systems comprising redox-inert metal ions.
钴(II)水络合物介导的过氧化氢活化:HOOOH和Co(II) -OOOH中间体在单线态氧生成中的可能作用
密度泛函理论(DFT)计算表明,[CoII(H2O)6]2+与两个H2O2分子反应形成[(H2O)4CoII(OOH)(H2O2)]+反应物配合物,根据配位-OOH和H2O2配体之间的相对取向,通过三种不同的途径分解。通过这些激活途径产生的活性中间体包括羟基(•OOH)/超氧化物(O2•-)自由基、单线态氧(1O2)和Co(III)种[(H2O)5CoIII(O)]+、[(H2O)4CoIII(OH)2]+和[(H2O)5CoIII(OH)]2+。Co(III)表现出从中等到强的氧化能力,这一直被忽视。值得注意的是,我们的DFT计算揭示了在[(H2O)4CoII(OOH)(H2O2)]+分解过程中可能形成三氧化氢(HOOOH)和Co(II) -OOOH中间体,并且这些瞬态物质的水解是生成1O2的途径。由于三种激活途径中的两种不涉及Co中心氧化态的变化,因此它们可能适用于含有氧化还原惰性金属离子的其他体系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


