Hyperreactive B cells instruct their elimination by T cells to curb autoinflammation and lymphomagenesis
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
B cell immunity carries the inherent risk of deviating into autoimmunity and malignancy, which are both strongly associated with genetic variants or alterations that increase immune signaling. Here, we investigated the interplay of autoimmunity and lymphoma risk factors centered around the archetypal negative immune regulator TNFAIP3/A20 in mice. Counterintuitively, B cells with moderately elevated sensitivity to stimulation caused fatal autoimmune pathology, while those with high sensitivity did not. We resolved this apparent paradox by identifying a rheostat-like cytotoxic T cell checkpoint. Cytotoxicity was instructed by and directed against B cells with high intrinsic hyperresponsiveness, while less reactive cells were spared. Removing T cell control restored a linear relationship between intrinsic B cell reactivity and lethal lymphoproliferation, lymphomagenesis, and autoinflammation. We thus identify powerful T cell-mediated negative feedback control of inherited and acquired B cell pathogenicity and define a permissive window for autoimmunity to emerge.
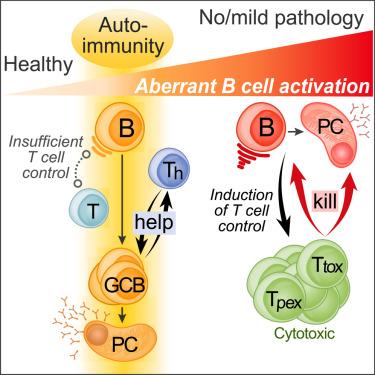
高反应性B细胞指示T细胞消除它们以抑制自身炎症和淋巴瘤形成
B细胞免疫具有向自身免疫和恶性肿瘤转变的固有风险,这两者都与增加免疫信号的遗传变异或改变密切相关。在这里,我们研究了自身免疫和淋巴瘤危险因素之间的相互作用,这些因素以小鼠的典型阴性免疫调节因子TNFAIP3/A20为中心。与直觉相反,对刺激适度升高敏感性的B细胞引起致命的自身免疫病理,而高敏感性的B细胞则没有。我们通过鉴定一种类似变阻器的细胞毒性T细胞检查点解决了这个明显的矛盾。细胞毒性由具有高内在高反应性的B细胞指示并直接针对B细胞,而反应性较低的细胞则幸免。去除T细胞控制恢复了内在B细胞反应性与致死性淋巴细胞增殖、淋巴瘤发生和自身炎症之间的线性关系。因此,我们确定了T细胞介导的对遗传和获得性B细胞致病性的强大负反馈控制,并定义了自身免疫出现的许可窗口。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


